| Delivery Systems for Respiratory Medications |
In addition to activities related to gas exchange, the respiratory system is responsible for a variety of other vital functions. One function of the upper airway is to assure that inspired gases are warmed and adequately humidified. Another function involves protecting the lungs by filtering inspired gas. RCPs need to understand basic concepts of humidification and aerosol systems in order to treat patients whose airway mechanisms are compromised.
Aerosol and humidity therapy are provided to maintain normal physiologic conditions and as therapy for pathologic conditions. One of the most important, but least understood, aspects of pulmonary care is the role of humidity therapy. Many care providers and most patients do not appreciate the role of hydration in liquifying secretions and facilitating the natural flow of mucus from the lower airways.
Pulmonary patients need adequate humidification of their inspired gases and controlled fluid balance, otherwise patients can become dehydrated. Dehydration can make secretions more viscous and inhibit the mucociliary escalator activity of the airways, making secretions difficult to dislodge. If this blocks functional gas flow through the distal airways, infections, atelectasis and other respiratory problems can easily occur.
Since humidification and humidity therapy are so important to respiratory well-being, you need to take a moment and review the basic physical principles of humidity. Humidity is essentially the water vapor in a gas. This water vapor can be described in several ways, as:
1. Absolute humidity ‑ The actual content of water vapor in a gas measured in milligrams per liter.
2. Potential humidity ‑ The maximum amount of water vapor a gas can hold at a given temperature.
3. Relative humidity ‑ The amount of water vapor in a gas as compared to the maximum amount possible, expressed as a percentage.
When these three are presented in equation form, their relationship becomes clearer:
Relative Humidity = Absolute Humidity x 100
Potential Water Vapor Content
When a gas or air becomes heated, it expands and more spaces are created between the molecules. The resulting warmer gases have greater capacity for “holding” more water vapor than do cooler gases. Therefore, potential humidity increases as the temperature of a gas increases. As a result, warm, humidified gas traveling through tubing tends to “rain‑out” water vapor as the gas cools and has a lower water‑carrying capacity. The table below illustrates the relationship of temperature and potential humidity:
Temperature |
Water Content |
Water Vapor Pressure |
|
|
C° |
F° |
(mg/L) |
(mm Hg) |
|
0 |
32.0 |
4.85 |
4.58 |
|
10 |
50.0 |
9.40 |
9.20 |
|
20 |
68.0 |
17.30 |
17.51 |
|
30 |
86.0 |
30.35 |
31.71 |
|
37 |
98.6 |
43.90 |
46.90 |
|
40 |
104.0 |
55.10 |
55.13 |
|
100 |
212.0 |
598.00 |
760.00 |
One of the more important gases found in air is water vapor. The amount of water vapor in the air can vary widely day-to-day, while gases like oxygen and nitrogen are present in relatively constant amounts. In general, water in vapor form is governed by gas laws and can be treated as a gas.
All bodies of water or moist organic bodies are capable of giving off water vapor. When water enters the air as a gas, the humidity of the air increases. The measure of how much water vapor is contained in the air is identified as the humidity level, and the factors determining the humidity include:
1. The availability of water. Clearly the air over a desert has less chance of picking up water vapor than the air over a lake.
2. Temperature is also a factor since the spacing of warmer air’s molecules allows water vapor’s molecules to fit more easily. Recalling the principles of Charles’ law, the volume of a gas increases as its temperature increases. If the number of molecules of the gases increases as the temperature rises, the humidity will not increase as much.
Summarizing, if there is water available there will be a specific amount of water vapor in the air at each ambient temperature. That amount equals the air’s total capacity for water vapor at that temperature. Air that contains its total capacity for water vapor at a specific temperature is said to be 100% saturated.
Amounts of water found in air are generally measured as grams per cubic meter of air (gm/ml) or as milligrams per liter of air (mg/1). These measurements can then be converted to moles of water by dividing by the gram molecular weight of water (which is 18). The total capacity of the air for water vapor is measured in milligrams per liter, with total capacity of the air for water vapor in milligrams per liter is referred to as the air’s potential water vapor. Table 1 identifies the various values of the potential water vapor content at several different temperatures.
THE POTENTIAL WATER VAPOR IS THE MAXIMUM THE AIR CAN HOLD AT A CERTAIN TEMPERATURE.
Table 1: Value of Potential Water Vapor Content |
|
|
Temperature |
Potential Water Vapor |
|
5°C |
6.8 mg/l |
|
10°C |
9.5 mg/l |
|
20°C |
17.3 mg/l |
|
25°C |
23.0 mg/l |
|
30°C |
30.4 mg/l |
|
37°C |
43.96 mg/l |
Air’s capacity for water cannot be met if there is not enough water available, and to discover the actual amount of water vapor present in the air it is necessary to measure the absolute humidity.
Relative humidity is another important measurement, and it represents the actual amount of water vapor in the air (absolute humidity) compared to the total possible water vapor content of the air at the given ambient temperature (potential water vapor). The measurement of relative humidity is expressed as a percentage (of saturation). For example, if the air contained only 17 mg/l of water vapor at 25°C, then it would not be totally saturated (see Table 1).
Calculating the relative humidity involves dividing the absolute humidity (actual water vapor content of the air) by the potential water vapor (maximum possible water vapor content of the air), and multiplying by 100 to convert the decimal percentage:
Relative Humidity = Absolute Humidity x 100
Potential Water Vapor Content
For the example above where the air had 17 mg/l of water vapor at 25°C:
Relative Humidity = 17/23 x 100
Therefore: Relative Humidity = 73.9%
Another factor to consider is the humidity deficit. For example, if the atmosphere’s relative humidity is less than 100%, the air of the atmosphere has what is referred to as a humidity deficit. If outside air at 20°C has 14 mg/l of water vapor, and needs to have 17.3 mg/l to be fully saturated, it is said to have a primary humidity deficit of 3.3 mg/l. To calculate the primary humidity deficit simply subtract the absolute humidity of the air from its potential water vapor at the appropriate temperature and the difference between the two is the primary humidity deficit:
Potential Water Vapor Content - Actual Water Vapor Content
The secondary humidity deficit also needs to be considered. This is the moisture deficit in the inspired air that the nose and upper airway needs to compensate for. When air is breathed into the nasal cavity and heated to body temperature, its potential water vapor rises to 44 mg/l that is the potential water vapor content of air at 37°C.
Therefore, unless the air of the atmosphere is at least 37°C and fully saturated, there exists a moisture deficit. For example, if the atmosphere’s air was 98.6°F (37°C) and the relative humidity 100%, people in such conditions would be very hot and sweaty! Luckily, inspired air is generally not that warm or humid, so most inspired air does have a secondary humidity deficit.
The secondary humidity deficit is calculated by subtracting the absolute humidity of the air from the potential water vapor content. The difference from the calculations for primary humidity deficits is that the potential water vapor content is always 44 mg/l, the potential water vapor of air at body temperature:
Secondary Humidity Deficit = 44 mg/l ‑ Absolute Humidity.
Therefore, if inspired air’s absolute humidity is 16 mg/l at 25°C before being warmed in the nasal cavity, there is a primary humidity deficit of:
Primary Humidity Deficit = 23 mg/l ‑ 16 mg/l = 7 mg/l
For this same absolute humidity for the atmosphere, the secondary humidity deficit is
Secondary Humidity Deficit = 44 mg/l ‑ 16 mg/l = 28 mg/l
Here are some other calculations for you to consider:
· If the absolute humidity of the same inspired air at 25°C were 23 mg/l, there would be no primary humidity deficit because the potential water vapor of air at 25°C is 23 mg/l (see Table above). There would still be a secondary humidity deficit of 21 mg/l because 44 mg/l minus 23 mg/l is 21 mg/l. This illustrates that at 100% relative humidity, it is still possible that the nasal cavity’s lining will have to supply moisture to the inspired air.
· You may sometime need to calculate a primary or secondary humidity deficit when you only know the relative humidity. To perform this calculation, you first need to convert the relative humidity into absolute humidity before calculating the humidity deficit. Remember the formula for relative humidity:
Relative Humidity = Absolute Humidity x 100
Potential Water Vapor Content
· When the temperature and relative humidity are known, you can look up the potential water vapor for the air temperature by using the Table shown above. Then substitute into the above formula the values for relative humidity and potential water vapor and calculate the absolute humidity. For example, the relative humidity at 10°C is 75% for the air in the atmosphere. Using the Table, you can see that the potential water vapor content at 10°C is 9.5 mg/l, so:
Relative Humidity = Absolute Humidity x 100
Potential Water Vapor Content
Absolute Humidity = 7.125 mg/l
This measurement of the absolute humidity can be used to calculate the primary and secondary humidity deficits of the same air. At 10°C, we know that the potential water vapor content is 9.5 mg/l, so the primary humidity deficit is:
9.5 ‑ 7.125 = 2.375 mg/l
The secondary humidity deficit for the same air would be:
Secondary Humidity Deficit = 44 - Absolute Humidity
44 ‑ 7.125 = 36.875 mg/l
To summarize:
· The primary humidity deficit occurs in the atmosphere and represents the difference between what humidity there is and what there could be.
· The secondary deficit occurs in the body and represents the difference between what humidity there is and what there needs to be at body temperature (37°C).
As discussed, water vapor acts in most ways like any other gas, air creates a partial pressure when it is in a mixture of gases. That partial pressure depends on the amount of water vapor present, which in turn depends on the temperature. However, water vapor differs from the behavior of other gases in the air since changes in the barometric pressure of the atmosphere under normal conditions do not have much impact on the partial pressure of water.
As a result, it is best to calculate the partial pressures of the other gases in the air after the partial pressure of water vapor has been determined—especially when measuring the air within the lungs. Inside the lungs, the partial pressure of water vapor is approximately 47 mm Hg. This value is relatively constant because the air entering the lungs is normally saturated and at 37°C.
By subtracting the partial pressure of the water vapor from the total atmospheric pressure, you will find what is referred to as the dry gas pressure. In the lungs,
Dry Gas Pressure = Atmospheric Pressure ‑ 47 mmHg
At one atmosphere (760 mmHg), the dry gas pressure would be:
Dry Gas Pressure = 760 ‑ 47 = 713 mmHg
As you have seen from the previous discussion, there are a number of reasons why humidity is an important aspect of the pulmonary system, including:
· It is needed to maintain normal bronchial hygiene
· It promotes functions of the normal mucociliary escalator
· It maintains the body’s vital homeostasis
· Without humidity the cleansing activities of the cilia could not function properly, and the nearly 100 ml of mucus secreted daily would become quite thick and tenacious.
· Without humidity the actual lung parenchyma would dry up, causing a loss of normal compliance which would restrict lung movement and reduce ventilation.
· If normal use of the route of humidification and recapture of water were lost, problems would most certainly present themselves. If the upper airway were bypassed or dry gases were inhaled, a series of adverse reactions could occur, including:
- Impairment of ciliary activity
- Slowing of mucus movement
- Inflammatory changes and possible necrosis of pulmonary epithelium
- Retention of thick secretions and encrustation
- Bacterial infiltration of mucosa (bronchitis)
- Atelectasis
- Pneumonia
As a result of the importance of maintaining humidity, humidity and aerosol therapy are also important, and their general goals are to:
1. Promote bronchial hygiene
2. Loosen dried and/or thick secretions
3. Promote a effective coughs to clear secretions
4. Provide adequate humidity in the presence of an artificial airway
5. Deliver adequate humidity when administering dry gases therapies
6. Delivering prescribed medications
There are a variety of factors to be considered when deciding to add humidity to dry gas therapies, including:
· patient’s age and ability to move normal secretions
· neuromuscular status
· recent or planned surgeries
· trauma
· disease conditions
The presence of any of these may impair the patient’s ability to cough and move secretions. Another problem may occur when patients develop very thick and abundant amounts of secretions that cannot be moved with normal muscle activity—making humidity or aerosol therapy necessary.
Primary indications for humidifying inspired gases include:
· Administration of medical gases
· Delivery of gas to the bypassed upper airway
· Thick secretions in non-intubated patients
Additional indications for warming inspired gases:
· Hypothermia
· Reactive airway response to cold inspired gas
Primary indications for aerosol administration:
· Delivery of medication to the airway
· Sputum inductions
Mucus generally comes from two sources: secretion from goblet cells and bronchial (mucous) glands. The goblet cells, which are distributed throughout the epithelium of the mucosa, synthesize and secrete mucus into the airway. The mucous glands, which are in the submucosa, are the greater source of mucus. Chronic irritation or disease can cause the number and size of goblet cells and mucous glands to increase, resulting in a larger and more viscous mucous blanket.
Ciliary activity, which moves the mucus, can be adversely affected if the mucous layer is changed. Changes in the ratio of gel to sol layer will affect the flow of mucus. A higher ratio, due either to a decrease in the watery sol layer or an increase in the viscous gel layer, could make the workload of the cilia too difficult to be effective. The cilia are capable of continuing to beat even if the workload increases, but only to a certain level. If the cilia become tangled in the thick mucus or are unable to penetrate the dense layer, the transport of the mucous blanket would stop, causing secretions to become retained in the respiratory tract.
Increases in the amount of watery sol fluid would also decrease the transport of mucus. The cilia must be able to extend through the sol layer to the gel layer to transport the mucus. Transport would be impaired if the thickness of the sol layer were to eliminate ciliary contact with the gel layer.
Other factors that can impede ciliary activity and the flow of mucus include:
· tobacco smoke
· local environmental conditions
· and pathology of the airway can impede clearance due to changes in the epithelium.
The effectiveness of humidifiers’ ability to adequately supply vapor to a gas depends on three factors: temperature, surface area and time.
Temperature increases cause increases in vapor pressure and potential humidity. The greater the surface area of water/gas contact and the longer time this contact takes place, the greater the number of water molecules that can enter the gas mixture. These principles are used by humidifiers to provide increased relative humidity to the gas.
Solution Gas
![]()

Figure 1. The blow-by humidifier.
The purpose of humidifiers is to deliver a gas with a maximum amount of water vapor content. These humidifiers may be heated or unheated, and the factors affecting the efficiency of humidification devices include:
· temperature
· time of exposure between gas and water
· and the surface area involved in the gas/water contact
As temperature rises, the force exerted by the water molecules increases, enabling their escape into the gas, adding to the humidity. Longer exposure of a gas to the water increases the opportunity for the water molecules to evaporate during the humidifier’s operation.
The pass-over type humidifier directs a dry gas source over a water surface area, which then flows to the patient. Because exposure area and time of contact is limited and it is not heated, this unit is not very efficient. These units are often used in incubators and in certain ventilators, although many times the use of a heated element is added to improve this humidification system (see Figure 1).
This type of system (see Figure 2) uses conduction to introduce the gas into the water below its surface. The gas passes through the liquid in the form of bubbles of various sizes. This is more effective because it increases exposure time and contact area. These units are called diffusers. The bubble or diffuser-type humidifiers are most commonly used.
The ability to hold water vapor falls as the temperature of gas falls, with room temperature’s relative humidity falling between 30 and 50%. Since a low water content actually reaches the patient, this type of humidification is not recommended for patients with an endotracheal tube, tracheostomy or tenacious secretions. Gas flow through these humidifiers affects humidity. The higher the flow rate of a gas, the less the exposure time to the airway. These units function best at a flow rate of 2 liters/minute and should not be run at greater than 6 liters/minute.
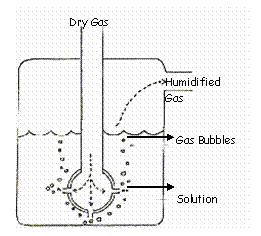 |
Figure 2. The bubble humidifier.
This type of humidifier actually forms an aerosol, but employs baffles to break the particles into small droplets, allowing them to evaporate. The gas is humidified further before it leaves the unit. The aerosol is formed by Bernoulli’s principle. A low pressure zone at the top of the water inlet tube draws water into the jet stream and the water is then aerosolized by the flow of gas.
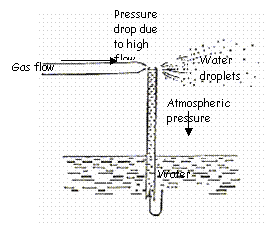
Bernoulli’s principle is employed as follows: Gas flows into the chamber through a restricted orifice, causing a high velocity flow which passes across a capillary tube that is immersed in water. The pressure drops around the opening of the capillary tube and water is forced up the capillary tube. The jet stream of air blows the liquid off in small particles as it reaches the top of the capillary tube.
The jet humidifier produces a high humidity output by employing Bernoulli’s principle to form an aerosol and using baffles to break up the particles.

Figure 4: Underwater jet humidifier
The underwater jet humidifier utilizes the principles of two other humidifiers: the bubble and the jet humidifiers. In the underwater jet, the restricted orifice and capillary tube are both below the surface of the water. The aerosolized gas then bubbles through the water, increasing surface area and water/gas contact time, increasing efficiency.
These humidifiers are indicated when it is necessary to deliver a humidified gas directly to the tracheobronchial tree (for example if the patient has a tracheostomy tube or an intubation tube), bypassing the natural humidification and heating system (nasal pharyngeal route). The gas must be delivered at 100% relative humidity at body temperature.
These devices (like the Cascade humidifier) incorporate a bubble pass‑over type of system. Gas is moved down a tower, passes through a grid that has a thin layer of water covering it and then over the warm water before being expelled out of the unit and more humidification takes place. This humidifier can deliver 100% relative humidity at various temperatures.
These humidifiers can go well above body temperature, creating the potential for tracheal burns or possible aspiration if tubing is not drained frequently. Wide bore tubing should always be used with these humidifiers, and be sure to humidify the gas during delivery.
It is important to remember that an aerosol is not the same as humidity. Humidity is water in a gas in molecular form, while an aerosol is liquid or solid particles suspended in a gas. Examples of aerosol particles can be seen everywhere: as pollen, spores, dust, smoke, smog, fog, mists, and viruses.
Aerosols can be created for therapeutic uses by physically shattering or shearing matter or liquid into small particles and dispersing them into a suspension. This can be accomplished by a variety of ways, including using gas jets, spinning disks, or ultra high frequency sound.
The particle size of an aerosol depends on the device used to generate it and the substance being aerosolized. Particles of this nature, between 0.005 and 50 microns, are considered an aerosol. The smaller the particle, the greater the chance it will be deposited in the tracheobronchial tree. Particles between 2 and 5 microns are optimal in size for depositing in the bronchi, trachea and pharynx.
Aerosol therapy is designed to increase the water content delivered to the pulmonary tree, and to deliver drugs to this area. Deposition location is of vital concern, and factors that affect aerosol deposition are aerosol particle size and particle number.
|
Table: Particle size and area of deposition. |
|
|
Particle Size in Microns |
Area of Deposition |
|
1 to 0.25 |
Minimal settling |
|
1 to 2 |
Enter alveoli with 95% deposition |
|
2 to 5 |
Deposit proximal to alveoli |
|
5 to 100 |
Trapped in nose and mouth |
Deposition of particles is also affected by:
· Gravity ‑ Large particles are deposited before smaller particles; and gravity affects large particles more than small particles, causing them to rain-out.
· Viscosity ‑ The viscosity of the carrier gas plays an important role in deposition. For example, if a gas like helium, which has a low viscosity and molecular weight, is used as a carrier gas, gravity will have more of an effect upon the aerosol. Helium is very light and hence can’t carry these particles well, leading to rain-out and early deposition.
· Kinetic activity ‑ As aerosolized particles become smaller, they begin to exhibit the properties of a gas, including the phenomenon of “Brownian movement.” This random movement of these small (below l mm) particles causes them to collide with each other and the surfaces of the surrounding structures, causing their deposition. As particle size drops below 0.1m, they become more stable with less deposition and are exhaled.
· Particle inertia ‑ Affects larger particles which are less likely to follow a course or pattern of flow that is not in a straight line. As the tracheobronchial tree bifurcates, the course of gas flow is constantly changing, causing deposition of these large particles at the bifurcation.
· Composition or nature of the aerosol particles ‑ Some particles absorb water, become large and rain-out, while others evaporate, become smaller and are conducted further into the respiratory tree. Hypertonic solutions absorb water from the respiratory tract, become larger and rain-out sooner. Hypotonic solutions tend to lose water through evaporation and are carried deeper into the respiratory tract for deposition. Isotonic solutions (0.9% NaCl) will remain fairly stable in size until they are deposited.
· Heating and humidifying ‑ As aerosols enter a warm humidified gas stream, the particle size of these aerosols win increase due to the cooling of the gas in transit to the patient. This occurs because of the warm humidified gas cooling and depositing liquid (humidity) upon the aerosol particles through condensation.
· Ventilatory pattern ‑ RCPs easily control this by simple observation and instruction. For maximum deposition, the patient must be instructed to:
1. Take a slow, deep breath.
2. Inhale through an open mouth (not through the nose).
3. At the end of inspiration, use an inspiratory pause, if possible, to provide maximum deposition.
4. Follow with a slow, complete exhalation through the mouth.
In many cases, aerosols are superior in terms of efficacy and safety to the same systemically administered drugs used to treat pulmonary disorders. Aerosols deliver a high concentration of the drugs with a minimum of systemic side effects. As a result, aerosol drug delivery has a high therapeutic index; especially since they can be delivered using small, large volume, and metered dose nebulizers.
| Delivery Systems for Respiratory Medications |