| Oral versus Inhaled Asthma Therapy |
Oral vs inhaled asthma therapy. Pros, cons and
combinations.
Fabbri LM, Piattella M, Caramori G, Ciaccia A.
In Drugs. 1996;52 Suppl 6:20-8.
Institute of Respiratory and Infectious Diseases, University of Ferrara, Italy.
A number of oral and inhaled drugs are available for the long term management of patients with persistent asthma, yet the disease continues to be associated with significant morbidity and mortality. Over the past years, inhaled glucocorticoids have become established as a cornerstone of maintenance therapy because of their demonstrated clinical efficacy, ability to reduce bronchial inflammation and good tolerability. Other inhaled drugs (e.g. sodium cromoglycate, nedocromil, long-acting beta2 agonists) also play a role in the long term treatment of patients with asthma. However, many patients (especially children and the elderly) find inhalers difficult to use, and poor inhalation technique can affect the amount of drug reaching the lungs and response to therapy. Oral drug administration is simple, but, until recently, oral asthma therapy has primarily consisted of sustained-release theophylline and glucocorticoids. Theophylline has a narrow therapeutic index, necessitating regular monitoring of serum drug concentrations, and long term oral glucocorticoid therapy is associated with potentially serious adverse events including osteoporosis with bone fracture. The recent development of orally administered leukotriene receptor antagonists (e.g. zafirlukast) and 5-lipoxygenase inhibitors (e.g. zileuton) offers novel mechanisms of action and potential solutions to compliance issues associated with regular administration of inhaled asthma therapy. These drugs have demonstrated efficacy as maintenance therapy in patients with asthma and, importantly, lack the adverse effects associated with long term systemic glucocorticoid therapy. Further clinical trials and the increasing use of these new therapies will help to establish the precise role of orally administered leukotriene receptor antagonists and 5-lipoxygenase inhibitors in the long term management of patients with asthma.
Early treatment with inhaled glucocorticoids gives optimal long-term control of asthma. In children, there is some evidence that delaying the initiation of such therapy may result in irreversible changes in the airways. Moderate doses of glucocorticoids by inhalation have proved to be safe for children, even young children, and even when given over long periods of time. In their study of 15 children aged 2 to 7 years given budesonide 200 microgram/day by inhalation, Volovitz et al. found the drug to be remarkably safe and effective for up to 5 years. The severity of asthma decreased in the first month of therapy (58% reduction in the number of days with asthma symptoms and 75% reduction in use of bronchodilators), and improvement was maintained. At the end of the trial, asthma recurred in 13 of the 15 patients enrolled, and budesonide was required to control the symptoms.
Budesonide did not suppress pituitary-adrenal function; there was no documented effect on 24-hour serum cortisol concentration, serum cortisol responses to corticotropin, or urinary cortisol excretion. Growth patterns — height, weight, and bone age — were normal throughout the treatment period for all patients. Larger doses have been shown to delay growth and skeletal maturation, but then so can asthma. No cataracts were seen (although cataracts have been reported with glucocorticoid inhalation therapy). The investigators concluded that “prolonged administration of budesonide in a relatively low dose of 200 microgram per day to young children with severe asthma is not only effective but also safe, as demonstrated by their normal linear growth and normal pituitary-adrenal function.” (Utiger RD. N Engl J Med. 1993; 329: 1731-1733. Volovitz B et al. N Engl J Med. 1993; 329: 1703- 1708.)
In the N Engl J Med, 1999, V 340:1005-1010, the following article was published:
Early Inhaled Glucocorticoid Therapy to Prevent Bronchopulmonary Dysplasia
Cynthia H. Cole, M.D., M.P.H., Theodore Colton, Sc.D., Bhavesh L. Shah, M.D., Soraya Abbasi, M.D., Brenda L. MacKinnon, R.N.C., Serkalem Demissie, M.P.H., and Ivan D. Frantz, M.D.
ABSTRACT
Background The safety and efficacy of inhaled glucocorticoid therapy for asthma stimulated its use in infants to prevent bronchopulmonary dysplasia. We tested the hypothesis that early therapy with inhaled glucocorticoids would decrease the frequency of bronchopulmonary dysplasia in premature infants.
Methods We conducted a randomized, multicenter trial of inhaled beclomethasone or placebo in 253 infants, 3 to 14 days old, born before 33 weeks of gestation and weighing 1250 g or less at birth, who required ventilation therapy. Beclomethasone was delivered in a decreasing dosage, from 40 to 5 µg per kilogram of body weight per day, for four weeks. The primary outcome measure was bronchopulmonary dysplasia at 28 days of age. Secondary outcomes included bronchopulmonary dysplasia at 36 weeks of postmenstrual age, the need for systemic glucocorticoid therapy, the need for bronchodilator therapy, the duration of respiratory support, and death.
Results One hundred twenty-three infants received beclomethasone, and 130 received placebo. The frequency of bronchopulmonary dysplasia was similar in the two groups: 43 percent in the beclomethasone group and 45 percent in the placebo group at 28 days of age, and 18 percent in the beclomethasone group and 20 percent in the placebo group at 36 weeks of postmenstrual age. At 28 days of age, fewer infants in the beclomethasone group than in the placebo group were receiving systemic glucocorticoid therapy (relative risk, 0.6; 95 percent confidence interval, 0.4 to 1.0) and mechanical ventilation (relative risk, 0.8; 95 percent confidence interval, 0.6 to 1.0).
Conclusions Early beclomethasone therapy did not prevent bronchopulmonary dysplasia but was associated with lower rates of use of systemic glucocorticoid therapy and mechanical ventilation.
From issue No. 248-249 (May/June, 1998) of Medical Sciences Bulletin
Recently, corticosteroids have been used as adjunctive therapy in certain infectious diseases, including acute viral laryngitis and H. influenzae meningitis. A study was conducted to determine if intranasal corticosteroids would decrease the duration and the severity of the symptoms of the common cold by lessening inflammation in the nasal cavity during the infection.
Two hundred adults, average age of 24 years, participated in this double-blind study. They were instructed to begin treatment with fluticasone propionate, a corticosteroid with broad antiinflammatory activity, or placebo, between 24 to 48 hours after cold symptoms appeared. The daily dose consisted of two puffs per nostril four times a day at equal intervals while they were awake, totaling 800 mg/day for 6 days. During the 21 days of the study, they kept a diary of the symptoms they were experiencing by assigning each a number representing its severity (0=absent, 3=severe). They also recorded adverse effects, time off from work or school due to the illness, and body temperature.
In general, fluticasone propionate treatment had no clinically recognizable effects on cold symptoms, although it significantly reduced nasal congestion and cough on some study days. For those infected with rhinoviruses, the treatment caused shedding of viable rhinoviruses, and viruses were found more often in the treated group then the placebo group (37% vs. 14%, p < 0.001). However, this did not impact the symptoms of the illness. This study showed that the symptoms were more severe in those subjects with positive cultures for S. pneumoniae, H. influenzae, or M. catarrhalis in the nasopharynx. No changes were observed in the colonization of these bacteria, and no effect on the severity of their symptoms was experienced by the subjects.
The study determined that using high doses of intranasal fluticasone propionate has no effect on the symptoms or duration of the common cold. In some cases, especially in patients with certain concomitant bacterial infections, symptoms were worse with treatment compared to placebo.
Puhakka T, Makela MJ, Malmstrom K, et al. The common cold: Effects of intranasal fluticasone propionate treatment. J Allergy Clin Immunol. 1998; 101: 726-31.
Mar. 27, 1998: Two studies published in British weekly medical journals present data on use of corticosteroids in chronic obstructive pulmonary disease and rheumatoid arthritis.
Inhaled fluticasone was compared with placebo in 281 COPD patients over a six- month period in a Lancet article (1998; 351: 773-80). Slightly more patients receiving placebo (37% vs. 32%) had at least one exacerbation, but exacerbations were classified as moderate or severe more frequently in the placebo group (86% vs. 60%). The authors conclude that inhaled corticosteroids may have a role in long-term treatment of COPD.
Reprints: J. Efthimiou, Glaxo Wellcome Research and Development, Greenford, Middlesex UB6 0HE, U.K.
An editorialist, commenting on the study (pp. 766-7), agreed that patients whose peak expiratory flow responds to steroids with increases of 15 L/min or more should be continued on such therapy.
Reprints: N. C. Barnes, Resp. Med., London Chest Hosp., London E2 9JX, U.K.
A meta-analysis in the British Medical Journal (1998; 316: 811-8) explores the utility of short-term, low-dose prednisolone in treatment of rheumatoid arthritis. Based on data from 10 published trials, the authors found that oral prednisolone had a marked effect over placebo on joint tenderness, pain, and grip strength. Compared with NSAID, prednisolone had a greater effect on joint tenderness and pain but not on grip strength. The group concludes that prednisolone doses of 15 mg or less per day may be used intermittently, especially when symptoms of rheumatoid arthritis are not otherwise controlled.
Reprints: P. C. Gotzsche, Nordic Cochrane Ctr., Rigshospitalet, Dept. 7112, Tagensvej 18 B, DK-2200 Copenhagen N., Denmark; p.c.gotzsche@cochrane.dk.
Accompanying editorialists were unconvinced (pp. 789-90) by the authors’ recommendations for more use of prednisolone. Explaining that the authors did not give full weight to the possibility of adverse drug reactions with steroids, the editorialists conclude, “Clinicians who encounter these adverse effects in day to day practice might be forgiven for adopting a more cautious stance than that adopted by the authors from the Nordic Cochrane Centre.”
Reprints: C. Cooper, U. Southampton, Southampton Genl. Hosp., Southampton, S016 6YD, U.K.
Apr. 21, 1999: Pharmacotherapy of asthma is addressed in several reports in the Journal of Allergy and Clinical Immunology (1999; 103; www1.mosby.com).
Therapeutic doses of fluticasone had little effect on the hypothalamic-pituitary-adrenal axis in a 28-day study (pp. 622-8). Mean plasma cortisol response to cosyntropin was similar among fluticasone, triamcinolone, and placebo and significantly less than in patients taking prednisone. Triamcinolone was statistically similar to placebo on most measures but reduced the area under the cortisol concentration/time curve.
Reprints: J. T. C. Li, Mayo Clinic Foundation, 200 1st St. SW, Rochester, MN 55905.
Bruno Balbi, MD, PhD; Maria Majori, MD; Stefano Bertacco, MD; Giuseppe Convertino, MD; Angelo Cuomo, MD; Claudio F. Donner, MD, FCCP and Alberto Pesci, MD, FCCP
Chest. 2000;117:1633-1637.)
© 2000 American College of Chest Physicians
Study objective: To investigate possible changes in cells and molecular mediators of airway inflammation following inhaled steroid treatment of stable COPD patients.
Design: Six-week open preliminary prospective study.
Setting: A university respiratory disease clinic.
Patients: Stable COPD patients with mild disease.
Intervention: Six-week treatment with inhaled beclomethasone (1.5 mg die).
Measurements: The levels of interleukin (IL)-8, myeloperoxidase, eosinophilic cationic protein and tryptase, and cell numbers in bronchial lavage specimens were determined, and the symptom score, the endoscopic bronchitis index, and functional parameters were recorded.
Results: After treatment there were significant reductions in the lavage levels of IL-8 ([mean ± SEM] 1,603.4 ± 331.2 vs 1,119.2 ± 265.3 pg/mL, respectively; p = 0.01) and myeloperoxidase (1,614.5 ± 682.3 vs 511.2 ± 144.2 µg/L, respectively; p = 0.05), in cell numbers (250.6 ± 27.7 vs 186.3 ± 11.5 cells x 103/mL, respectively; p = 0.04), neutrophil proportion (59.7 ± 14.3% vs 31.5 ± 10.1%; p = 0.01), symptom score (4.5 ± 0.6 vs 1.4 ± 0.5; p = 0.01), and bronchitis index (8.5 ± 0.8 vs 5.5 ± 0.7; p = 0.007).
Conclusions: In stable patients with COPD, inhaled steroid treatment may induce changes on some cellular and molecular parameters of airway inflammation.
Tradename Singulair
Manufacturer Merck & Co., Inc
Treatment Class Anti-inflammatory, Antiallergic, and Immunologic
Indication Asthma
From issue No. 246 (March, 1998) of Medical Sciences Bulletin
Montelukast was recently approved by the FDA for the prevention and chronic treatment of asthma in adults and children aged 6 years and older. It is the third anti-leukotriene agent (the first two being zafirlukast and zileuton) to be approved for treatment of asthma and the only anti-leukotriene approved for use in children.
The prevalence of asthma, a serious and common chronic disease, is increasing worldwide, especially among children. Asthma is defined as a chronic inflammatory disorder of the airways, marked by reversible airway narrowing and increased airway responsiveness to a variety of stimuli. The recommended therapy for asthma, as outlined in the guidelines of the National Asthma Education and Prevention Program, varies according to asthma severity. In all but mild intermittent asthma, for which the recommended therapy is a short-acting inhaled beta2 agonist for exacerbations, the cornerstone of asthma treatment is anti-inflammatory therapy.
How It Works
Montelukast is a potent and specific
antagonist of the cysteinyl leukotriene receptor, known as the CysLT1 receptor,
and thus inhibits the physiologic action of leukotriene D4 at this receptor.
The cysteinyl leukotrienes B4, C4, and D4 were formerly known collectively as
the slow-reacting substance of anaphylaxis (SRS-A). These compounds are
released from inflammatory cells, including mast cells and eosinophils, and
contribute in several ways to the pathophysiology of asthma. Their effects
include bronchoconstriction, increased airway responsiveness, enhanced mucus
secretion, increased vascular permeability leading to airway edema, and
decreased action of respiratory cilia.
After oral administration, montelukast is rapidly absorbed, with mean oral bioavailability of 64%. The absorption of montelukast is not influenced by the ingestion of food. Maximum plasma concentrations are reached 3 to 4 hours after administration of a 10-mg film-coated tablet and 2 to 2.5 hours after administration of a 5-mg chewable tablet. Montelukast is eliminated predominantly by metabolism followed by biliary excretion. The mean plasma half-life in young adults ranges from 2.7 to 5.5 hours. There is minimal accumulation of parent drug at steady state.
Clinical Tips
The clinical utility and safety of
montelukast have been studied in large American and multinational clinical
trials. Montelukast has been evaluated in adult patients with asthma of varying
severity, including mild and moderate asthma, in addition to exercise-induced
bronchospasm and aspirin-sensitive asthma. Clinical trials in children 6 to 14
years of age have assessed the efficacy and safety of montelukast in pediatric
patients with mild to moderate asthma and those with exercise-induced
bronchospasm.
Endpoints examined in large-scale clinical trials enrolling adult patients with mild persistent asthma not controlled with beta agonist included both objective and subjective asthma parameters as well as asthma outcomes such as frequency of asthma exacerbations and attacks and the need for rescue corticosteroid therapy. Montelukast produced rapid improvements in asthma signs and symptoms, with decreases in beta-agonist use and improved asthma symptoms evident after the first dose. After 12 weeks of therapy with montelukast 10 mg once daily at bedtime, forced expiratory volume in 1 second (FEV1) had improved significantly and beta-agonist use had fallen significantly as compared with results for patients receiving placebo. In addition, significant improvements relative to placebo were recorded for montelukast-treated patients in daytime symptom scores, frequency of nocturnal awakenings, quality-of-life measures, and several asthma outcomes, notably frequency of asthma exacerbations and attacks and the need for oral corticosteroid rescue therapy. No tolerance to the treatment effect of montelukast has been noted in nonplacebo-controlled extension studies of up to 1 year in duration.
The suitability of once-daily administration of montelukast 10 mg was evaluated in a small group of patients with exercise-induced bronchospasm. Compared with patients receiving placebo, patients treated with montelukast showed significant reductions in the fall in FEV1 after exercise. This protection was present at the end of the dosing interval, namely 20 hours after the previous day’s bedtime dose of montelukast, and persisted throughout the 12-week treatment period, with no evidence of tolerance developing. Among children, montelukast 5 mg once daily also inhibited exercise-induced bronchoconstriction at the end of the dosing interval.
In patients receiving an inhaled corticosteroid, the addition of montelukast to the treatment regimen produced additional benefit, as measured by significant improvement in FEV1 relative to patients receiving inhaled beclomethasone alone. In another double-blind, placebo-controlled trial, addition of montelukast permitted tapering of the inhaled corticosteroid dose by a mean of 47% over 12 weeks of treatment compared with 30% in the placebo group. Fully 40% of montelukast-treated patients were able to taper completely off corticosteroids during the treatment period, more than in the placebo group (29%).
In a 4-week study of aspirin-sensitive asthmatics, 87% of whom were receiving oral or inhaled corticosteroids or both, the addition of montelukast to treatment caused significant improvements in FEV1 and beta-agonist use. Moreover, patients in the montelukast group had fewer days with asthma exacerbations than those in the placebo group and reported significant improvement in global evaluations and quality-of-life assessments.
Montelukast has been studied in one large, double-blind, placebo-controlled clinical trial of children aged 6 to 14 years. Results for pediatric patients receiving 5 mg montelukast once daily were similar to those for adult asthmatics, with significant improvement recorded in both objective and subjective asthma parameters, as well as improvement in parental global evaluations and asthma exacerbation rates. Merck has committed to study the effect of chronic, long-term administration of montelukast on linear growth among pediatric patients.
Clinical trial results suggest that montelukast can be administered as controller therapy for patients with mild, persistent asthma whose symptoms are not controlled with as-needed beta agonist. In addition, patients with moderate to severe asthma who are receiving inhaled or oral corticosteroids, or both, may benefit from addition of montelukast to the treatment regimen. However, at present, insufficient data are available for the manufacturer to make specific recommendations for these uses.
Adverse events among patients receiving montelukast occurred with frequency similar to that among patients in the placebo groups. Montelukast use does not appear to be associated with rises in serum transaminase levels, as has been reported for other anti-leukotriene agents.
Montelukast shows a dose response, with the 10-mg dose identified as the minimal dose producing maximum clinical response in adults. There is no evidence of dose-related toxicity, as early studies assessed doses as high as 200 mg daily for several weeks without evidence of tolerability or safety problems. The recommended dose in adolescents and adults 15 years of age and older is 10 mg once daily in the evening. The pediatric dose for patients 6 to 14 years of age is 5 mg once daily in the evening, administered as a chewable tablet.
1. National Asthma Education and Prevention Program. Practical Guide for the Diagnosis and Management of Asthma. National Institutes of Health; National Heart, Lung, and Blood Institute. October 1997. NIH Publication 97-4053.
2. Singulair (montelukast sodium)-Product Package Insert, February 1998.
3. DeLepeleire I, Reiss TF, Rochette F, et al. Montelukast causes prolonged, potent leukotriene D4-receptor antagonism in the airways of patients with asthma. Clinical Pharmacology and Therapeutics. 1997;61:83-92.
4. Holgate ST, Bradding P, Sampson AP. Leukotriene antagonists and synthesis inhibitors: new directions in asthma therapy. Journal of Allergy and Clinical Immunology. 1996;98:1- 13.
5. Knorr BA, Matz J, Bernstein JA, et al. Montelukast (MK-0476) improves asthma over a 2 month treatment period in 6- to 14- year olds. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):A664. Abstract.
6. Kuna P, Malmstrom K, Dahlen SE, et al. Montelukast (MK-0476), a CysLT1 receptor antagonist, improves asthma control in aspirin-intolerant asthmatic patients. American Journal of Respiratory and Critical Care Medicine. 1997;155(4):A975. Abstract.
7. Reiss TF, Chervinsky P, Edwards T, et al. Montelukast (MK-0476), a CysLT1 receptor antagonist, improves the signs and symptoms of asthma over a 3 month treatment period. European Respiratory Journal. 1996;9(23):273S. Abstract.
8. Reiss TF, Sorkness CA, Stricker W, et al. Effects of montelukast (MK-0476), a potent cysteinyl leukotriene receptor antagonist, on bronchodilation in asthmatic subjects treated with and without inhaled corticosteroids. Thorax. 1997;52:45-48.
Asthma Update: Part II. Medical Management
July 16, 1998: This morning’s New England Journal of Medicine (1998; 339) sheds light on appropriate therapy of exercise-induced asthma.
Long-term treatment with salmeterol protected against exercise-induced asthma, but continued administration reduced the length of time for which the drug is effective (pp. 141-6).
In patients performing cycle ergometry while breathing frigid air, salmeterol’s effectiveness at 30 minutes after an inhaled dose was maintained throughout a one-month study. However, the drug’s effect at nine hours after a dose declined over the 30-day period.
Reprints: E. R. McFadden, Jr., Pulmonary and Critical Care Medicine, U. Hosp. of Cleveland, 11100 Euclid Ave., Cleveland, OH 44106.
In 110 patients ages 15-45 years with mild asthma, montelukast 10 mg daily at bedtime protected against exercise-induced asthma over a 12-week study period. Compared with placebo, the leukotriene modifier improved the area under the FEV1 curve, the maximal decrease in FEV1 after exercise, and the time to return from the maximal decrease in FEV1 to pre-exercise levels. Adverse effects were similar between the groups, and patients receiving montelukast did not experience tolerance to the medication or rebound worsening of lung function after discontinuation. However, one fourth of the patients did not respond to montelukast therapy.
Reprints: T. F. Reiss, Merck and Company, P.O. Box 2000, Rahway, NJ 07065.
Editorialists commenting on the studies warn that more head-to-head comparisons of active asthma therapies are needed. “Only patients with easily controlled, stable asthma were studied,” they explain. “Whether the long-term control of exercise-induced symptoms provided by montelukast is similar or superior to that provided by other leukotriene modifiers or an optimally administered inhaled glucocorticoid remains to be tested in patients with mild, moderate, or severe asthma. Until more direct comparisons are available, physicians and their patients must decide for themselves which among an expanding range of therapeutic options to use.”
Reprints: J. Hansen-Flaschen, U. Pennsylvania Sch. of Med., Philadelphia, PA 19104.
January 18, 2000:
A new study concludes that Merck & Co.’s drug Singulair (montelukast) offers longer-lasting protection against exercise-induced asthma flare-ups, compared to the commonly used, and older, drug Serevent (salmeterol), from Glaxo Wellcome.
The study is in the Annals of Internal Medicine (2000;132:97-104).
Tradename Ultair
Manufacturer SmithKline Beecham
Treatment Class Respiratory
Indication Asthma
Reprinted from the November 1996 issue [Med Sci Bull. 1996;19(3):8] of Medical Sciences Bulletin
Asthma is a chronic inflammatory disease of the airways that is complicated by episodes of acute inflammation. Even patients with mild disease show airways inflammation, including infiltration of the mucosa and epithelium with activated T cells, mast cells, and eosinophils. T cells and mast cells release cytokines that promote eosinophil growth and maturation and the production of IgE antibodies, and these, in turn, increase microvascular permeability, disrupt the epithelium, and stimulate neural reflexes and mucus-secreting glands. The result is airway hyperreactivity, bronchoconstriction, and hypersecretion, manifested by wheezing, coughing, and dyspnea.
Traditionally, asthma has been treated with oral and inhaled bronchodilators. These agents help the symptoms of asthma, but do nothing for the underlying inflammation. Recognition during the last 10 years of the importance of inflammation in the etiology of asthma has led to the increased use of corticosteroids. However, many patients continue to suffer from uncontrolled asthma. Now the FDA has approved the first of a new class of antiasthma drugs — the leukotriene inhibitors and antagonists — with the potential to interfere with the initial steps in the inflammatory cascade.
We first reported on leukotrienes in MSB back in 1979, when the so-called “slow reacting substance of anaphylaxis” was identified as an arachidonic acid derivative and given the name “leukotriene C.” Since that time, scientists have determined that the leukotrienes (of which there are A, B, C, D, and E subtypes) play a crucial role in asthma. They cause airways smooth muscle spasm, increased vascular permeability, edema, enhanced mucus production, reduced mucociliary transport, and leukocyte chemotaxis.
Like the related prostaglandins, leukotrienes are synthesized from arachidonic acid in the cell membrane. Arachidonic acid in mast cells, macrophages, monocytes, eosinophils, and basophils is released from membrane phospholipids by the activation of phospholipase A2. After its release, arachidonic acid undergoes metabolism via two major pathways: the cyclooxygenase pathway (which produces various prostaglandins and thromboxanes) and the 5-lipoxygenase pathway (which produces leukotrienes). The prostaglandins, thromboxanes, and leukotrienes are known collectively as eicosanoids.
There are a number of anti-leukotrienes under investigation that either block leukotriene receptors or prevent leukotriene synthesis by blocking the enzyme 5-lipoxygenase (just as aspirin and the nonsteroidal anti-inflammatory agents block the other enzyme — cyclooxygenase — involved in arachidonic acid metabolism). The leukotriene inhibitors are a varied lot: some block 5-lipoxygenase directly, some inhibit the protein that “presents” arachidonate to 5-lipoxygenase, and some displace arachidonate from its binding site on the protein. The leukotriene antagonists, by contrast, block the receptors themselves that mediate airways hyperreactivity, bronchoconstriction, and hypersecretion.
The market for the new leukotriene inhibitors and antagonists is in the billions of dollars. An estimated 13 million Americans have asthma, and many are not controlled with available bronchodilators and corticosteroids. Indeed, asthma mortality has risen 40% since 1982. Abbott, Merck, and SmithKline Beecham all have anti-leukotrienes in final clinical trial, and Zeneca’s zafirlukast (Accolate) was approved in late September. Abbott’s zileuton (Zyflo) was the first leukotriene to be reviewed by the FDA. It was rejected in October 1995 because of adverse effects on liver function tests, but Abbott refiled an application in June, and an FDA advisory committee has recommended the drug for approval with the suggestion that liver function be carefully monitored. SmithKline Beecham’s pranlukast (Ultair) is a leukotriene receptor antagonist licensed from Ono Pharmaceutical and approved for marketing in Japan. Merck’s montelukast (Singulair) is a long-acting
agent that will be the subject of an NDA filing during the first part of 1997. A number of additional drugs are under investigation, including the leukotriene antagonists pobilukast, tomelukast, and verlukast, and several inhibitors of leukotriene synthesis. (Holgate ST et al. J Allergy Clin Immunol. 1996;98:1-13. Spector SL. Annals of Allergy, Asthma, Immunol. 1995;75:473- 474. Additional information from the manufacturers.)
Aug 28, 1998: An editorial in the Journal of Allergy and Clinical Immunology (1998; 102: 170-2) assesses the place of leukotriene modifiers in asthma therapy. Responding to research indicating that the investigational agent pranlukast may attenuate allergen-induced early- and late-phase pulmonary responses, the editorialist notes, “The challenge for the future will be to show that the leukotriene modifiers have significant effects on reducing airway inflammation and disease progression.... If this is accomplished, the leukotriene modifiers may become one of the preferred long-term controller therapies for asthma.”
The author names these potential applications of the orally administered agents:
New research could help physicians tailor asthma therapy for children, from the Journal of Allergy & Clinical Immunology
Researchers
have identified specific asthma characteristics
in children that could help determine the type of asthma treatment they will best respond to.
These findings were published in the February 2005 Journal of Allergy &
Clinical Immunology (JACI). The JACI is the peer-reviewed, scientific journal
of the American Academy of Allergy, Asthma
and Immunology (AAAAI).
“Parents of children with asthma often ask: Is there a good way to determine
what medication will work best in my child?” said Stanley J. Szefler, MD,
FAAAAI. “These findings begin to address this question by taking a step toward
enabling clinicians to better individualize asthma therapy.”
Dr. Szefler and colleagues from the National Heart, Lung, and Blood Institute’s
(NHLBI’s) Childhood Asthma Research and
Education (CARE) Network found specific differences in responses to the inhaled
corticosteroid, fluticasone, and the leukotriene receptor antagonist,
montelukast, in children with mild-to-moderate persistent asthma.
Inhaled corticosteroids are anti-inflammatory medications that go directly into
the lungs, reducing inflammation in the airways. Leukotriene receptor antagonists
treat asthma differently by blocking substances in the lungs called
leukotrienes, which cause narrowing and swelling of the airways. While both
medications are considered effective daily treatments for long-term care and
prevention of exacerbations in patients of all ages with persistent asthma
(those who have symptoms at least two days a week or two nights a month), the
National Asthma Education and Prevention Program asthma treatment guidelines
list inhaled corticosteroids as the preferred treatment, with leukotriene
modifiers one of several alternative therapies.
“There is increasing evidence that children respond differently to the various
treatment options for asthma,” noted James
Kiley, PhD, director of the NHLBI Division of Lung Diseases. “If we can
pinpoint in advance which children will do better with a certain type of
therapy, we can improve their lives more quickly and save them the risk of
trying medications that are less effective for them. This study adds important
information for identifying which children are more likely to respond well to
inhaled corticosteroids.”
Researchers administered fluticasone and montelukast separately for 8 weeks to
126 children (ages 6 to 17 years) with mild-to-moderate persistent asthma.
During the course of the study, researchers evaluated the children’s lung
function in response to each therapy to determine which medication produced the
most favorable response.
The researchers reported on the percentage of children who improved lung
function by 7.5% or greater based on a standard test. They found:
Researchers
noted that children whose asthma improved with inhaled
corticosteroids had low pulmonary function and elevated markers of allergic
inflammation at baseline. On the other hand, the children whose asthma was improved only by the leukotriene
receptor antagonist were younger in age and had a shorter duration of the disease.
Based on these findings, researchers recommend that children with
mild-to-moderate persistent asthma who have low lung function and/or elevated
signs of allergic inflammation be treated daily with inhaled corticosteroids.
Their findings also suggest that, in those children who have no elevated signs
of allergic inflammation, a therapeutic trial of either medication can be
conducted to determine which works best.
This study was published in the peer-reviewed, scientific journal of the American Academy of Allergy, Asthma & Immunology (AAAAI). To request a copy, please contact John Gardner (jgardner@aaaai.org) at (414) 272-6071. For more information and access to the archive of past JACI news releases, visit the Media Center of the AAAAI Web site, http://www.aaaai.org.
Tradename Serevent Inhalation Aerosol
Manufacturer Glaxo Wellcome
Treatment Class Respiratory
Indication Asthma
From the June 1994 issue of Medical Sciences Bulletin, published by Pharmaceutical Information Associates, Ltd.
The Food and Drug Administration (FDA) recently granted marketing approval for salmeterol xinafoate (Serevent Inhalation Aerosol/Glaxo). The product is the first long-acting inhaled bronchodilator to reach the US market. It is indicated for twice-daily use in maintenance treatment of asthma and prevention of bronchoconstriction in patients aged 12 years or older who have obstructive airway disease.
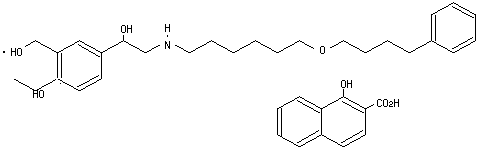
Serevent has a large potential market: an estimated 12 million Americans have asthma. A chronic condition, asthma is characterized by symptoms ranging from occasional wheezing and coughing to severe shortness of breath and tightening in the chest. Asthma sufferers often wake up during the night because of nocturnal symptoms. Until now, bronchodilator therapy meant having to inhale doses every 4 to 6 hours; thus, it was not possible get a full night’s protection. Serevent represents a significant advance, because a single dose provides 12-hour protection.
Chemically, Serevent is a racemic form of the 1-hydroxy-2-naphthoic acid salt of salmeterol, a highly selective beta2-adrenergic bronchodilator. The aerosol formulation consists of the active drug in a mixture of trichlorofluoromethane and dichlorodifluoromethane with lecithin.
Salmeterol is at least 50 times as selective for beta2-adrenoceptors as albuterol. Like other beta2-adrenoceptor agonists, salmeterol stimulates the intracellular enzyme adenyl cyclase, which catalyzes the conversion of adenosine triphosphate to cyclic adenosine monophosphate (cAMP). Accumulation of cAMP relaxes bronchial smooth muscle and inhibits the release of mediators of immediate hypersensitivity, especially from mast cells. In humans, salmeterol inhibits early- and late-phase responses to inhaled allergens, an effect that lasts more than 30 hours. Single doses of the drug attenuate allergen-induced bronchial hyperresponsiveness; however, the clinical relevance of this anti-inflammatory action has not been established.
Because salmeterol acts locally in the lungs, plasma drug concentrations are not clinically relevant. After administration of therapeutic doses, systemic concentrations may be very low or nondetectable. Nevertheless, in healthy volunteers, administration of 1 mg of drug produced peak plasma concentrations of about 650 pg/mL at about 45 minutes. The estimated terminal elimination half-life was 5.5 hours.
Salmeterol undergoes extensive hydroxylation and is eliminated primarily in the feces. The drug is 94% to 98% bound to plasma proteins.
In single-dose trials, salmeterol produced effective bronchodilation, defined as an increase of more than 15% in 1-second forced expiratory volume (FEV1), within 10 to 20 minutes after administration of a 42-µg dose. Peak therapeutic improvement occurred about 180 minutes after administration, and in most cases the improvement remained clinically significant for 12 hours.
Serevent has been used in more than 50 countries. Clinical trials have tested the product in more than 35,000 patients. It has been shown to significantly increase mean morning peak expiratory flow rate and the mean percentages of days without symptoms and of nights without awakenings and to decrease the need for rescue medications.
In maintenance therapy, the recommended dosage is 42 µg (two inhalations) of drug twice a day (morning and evening). For prevention of exercise-induced bronchoconstriction, patients should take two inhalations 30 to 60 minutes before exercising. (Note: patients using Serevent for maintenance therapy should not take additional doses to prevent exercise-induced bronchoconstriction, and the drug should not be used to treat acute symptoms of asthma). Serevent may be used with or without concomitant corticosteroid therapy. However, if patients are using corticosteroids, they should not stop or reduce that medication just because they feel better — salmeterol and corticosteroids have different actions, salmeterol inhibiting airway constriction and corticosteroids suppressing inflammation. Patients may use short-acting beta-agonists intermittently while receiving Serevent maintenance therapy, but they should discontinue maintenance use of any short-acting bronchodilators. They should also be alert for an increasing need for short-acting drugs, which may be a sign of destabilized asthma.
Side effects are similar to those reported for other widely used inhaled bronchodilators. The most frequently reported are tremors, headaches, and coughs. Other possible adverse reactions include tachycardia, palpitations, immediate hypersensitivity reactions, nervousness, and paradoxical bronchospasm. Use of Serevent is contraindicated for patients with hypersensitivity to any component of the product. Current evidence indicates that dose alteration is not necessary for elderly patients. Use in infants or children less than 12 years old or in pregnant or nursing women has not been studied. Concomitant use of monoamine oxidase (MAO) inhibitors may potentiate the effects of Serevent.
Important concerns regarding the safety of salmeterol have been raised internationally. There was a small, statistically nonsignificant increase in asthma-related deaths among patients treated with salmeterol compared with those taking albuterol. (Castle W et al. Br Med J. 1993; 306: 1034- 1037.) Others have reported cases of sudden respiratory arrest in otherwise healthy young asthmatics treated with salmeterol. (Clark CE et al. Respir Med. 1993; 87: 227-228.) Further experience with the drug is needed to confirm whether this is a clinically important problem with salmeterol.
Serevent Inhalation Aerosol is available in 13-g canisters containing 120 metered actuations (each delivering 25 µg salmeterol base from the valve and 21 µg from the actuator). Also available are 6.5-g canisters containing 60 metered actuations.
April 19, 1999: A recent study concludes that Glaxo Wellcome Inc.’s Serevent® (salmeterol xinafoate) Inhalation Aerosol is effective in improving lung function in patients with chronic bronchitis and emphysema (chronic obstructive pulmonary disease (COPD)).
The study is in the journal Chest (April, 1999).
Feb. 17, 1998: Tolerance to the bronchoprotective effect of salmeterol against methacholine- induced bronchoconstriction develops after only the second dose of the long- acting beta2 agonist. The study of 10 patients with mild asthma is in Annals of Allergy, Asthma, and Immunology (1998; 80: 31-4).
Concerns about regular use of beta agonists in patients with asthma have led researchers to analyze methacholine-induced bronchoconstriction in patients taking salmeterol. The current study indicates that, after one dose of salmeterol, the amount of methacholine needed to produce a 20% fall in FEV1 was significantly greater than the amount required after two doses, indicating development of tolerance.
Reprints: D. E. Drotar, Dept. of Med., Royal U. Hosp., Saskatoon, SK S7N 0W8, Canada.
Mar. 16, 1998: In the Journal of Allergy and Clinical Immunology (1998; 101: 188-95), researchers report that salmeterol improved quality of life indicators in patients whose conditions were not well-controlled with daily inhaled corticosteroids. On the disease-specific Asthma Quality of Life Questionnaire, global scores improved, as did individual domains (activity limitations, asthma symptoms, emotional function, and environmental exposure).
Patients taking salmeterol had improved clinical measures of efficacy, the paper reports, as well as a decreased requirement for as-needed albuterol. The 506 patients were randomly assigned to salmeterol 42 mcg or placebo delivered using a metered dose inhaler. Improvements were found in FEV1, morning and evening expiratory flow, and asthma symptoms.
Reprints: J. P. Kemp, Allergy & Asthma Med. Grp. and Res. Ctr., APC, 9610 Granite Ridge Dr., Suite B, San Diego, CA 92123.
Tradename Brethine (CibaGeneva Pharmaceuticals), Bricanyl (Aventis)
Manufacturer CibaGeneva Pharmaceuticals, Aventis
Treatment Class Respiratory
Indication bronchospasm due to asthma, bronchitis
FOR
IMMEDIATE RELEASE
November 13, 1997
FOOD AND DRUG
ADMINISTRATION
Broadcast Media: (301) 827-3434
Consumer Hotline: (800) 532-4440
Source: FDA MedWatch Program
Dear Colleague:
The Food and Drug Administration (FDA) would like to call to your attention concerns about subcutaneous administration, via infusion pump, of terbutaline sulfate for the treatment and prevention of preterm labor (tocolytic therapy).
Terbutaline sulfate, in various dosage forms, has been approved by FDA for the treatment of asthma. Adequate data establishing the safety and effectiveness of the use of terbutaline as a tocolytic agent have not been submitted to FDA. Thus, the use of terbutaline sulfate to treat preterm labor is an unapproved or “off-label” use. The only drug product currently approved for tocolytic therapy is ritodrine hydrochloride injection (Yutopar Intravenous Injection, manufactured by Astra), and it is approved for intravenous use only.
FDA is concerned about the promotion and increasingly widespread use of subcutaneous terbutaline delivered by infusion pump for the treatment/prevention of preterm labor. The approved labeling for terbutaline sulfate injection (Brethine, manufactured by CibaGeneva Pharmaceuticals, and Bricanyl, marketed by Hoechst Marion Roussel), states that the drug should not be used for the management of preterm labor. Infusion pumps are separately reviewed by FDA, and they are not labeled for subcutaneous administration of terbutaline.
Based on information available to the Agency, as well as a review of the published literature, it is clear that the demonstrated value of tocolytics in general is limited to an initial, brief period of treatment, probably no more than 48-72 hours. No benefit from prolonged treatment has been documented. In addition, the safety of long-term subcutaneous administration of terbutaline sulfate, especially on an outpatient basis, has not been adequately addressed.
Published reports on the safety of this use are seriously hampered by methodologic inadequacies. It appears that women receiving continuous subcutaneous terbutaline sulfate infusions experience side-effects and complications similar to those experienced by women receiving terbutaline and other beta-sympathomimetics intravenously. Complications include chest pain, tachycardia, dyspnea, and pulmonary edema. At least one maternal death occurred during outpatient use of a continuous infusion of terbutaline sulfate by subcutaneous pump. The impact of long-term use on maternal glucose metabolism and the risks of prolonged exposure of the fetus are largely unknown.
In June 1995, the American College of Obstetrics and Gynecology (ACOG) issued Technical Bulletin Number 206 which addresses preterm labor and, specifically, the use of tocolytic agents to manage uterine contractions. This bulletin notes that intermittent administration of subcutaneous terbutaline has been proposed as an alternative to oral maintenance therapy in certain patients. As stated in the Technical Bulletin, the ACOG found no clinical evidence to support the efficacy of this approach. Further, the bulletin states, “To date, no studies have convincingly demonstrated an improvement in survival or any index of long-term neonatal outcome with the use of tocolytic therapy. On the other hand, the potential damages of tocolytic therapy to the mother and neonate are well documented.”
In the absence of data establishing the effectiveness and safety of the drug/device, FDA is alerting practitioners, home healthcare agencies, insurance carriers, and others that continuous subcutaneous administration of terbutaline sulfate has not been demonstrated to be effective and is potentially dangerous. FDA is investigating the promotional activities of companies providing tocolytic therapy services. We encourage healthcare professionals to report adverse events associated with the use of terbutaline sulfate as a tocolytic agent to the FDA’s MedWatch program at 1-800-FDA-1088/FAX 1-800-FDA-0178. This is a voluntary system of reporting adverse events and product problems to FDA.
If you have comments and concerns about this issue, please contact the FDA Office of Health Affairs, Medicine Staff, telephone number (301) 443-5470.
Tradename Various
Manufacturer Various
Treatment Class Respiratory
Indication Asthma
Pediatrics, March, 1998 by Bruce G. Bender, David N. Ikle, Thomas DuHamel, David Tinkelman
The choice of medications used to treat pediatric asthma is routinely based on a number of considerations, including the child’s asthma history and symptoms and each medication’s relative benefits and risks. The side-effect potential of any medication is a necessary consideration that can contribute to the choice of an alternative medication. Theophylline, for several decades the most frequently selected asthma treatment, has been widely replaced as the first-choice chronic asthma medication in favor of aerosolized antiinflammatory drugs such as cromolyn, nedocromil, and corticosteroids. Several factors, including its potential for medical and psychological side effects, have led physicians away from theophylline treatment of pediatric asthma. The list of theophylline’s reported psychological side effects includes impaired attention,[1] impulse control,[2] motor steadiness[3], visual-spatial planning,[4] memory,[2,5] and school performance.[6] Half of the parents surveyed stated that their asthmatic children became restless and overactive when treated with theophylline.[7] In contrast, inhaled corticosteroids have been reported to cause relatively fewer side effects.[8,9] Case reports of hyperactive, aggressive, and oppositional behavior after inhaled corticosteroid treatment have been recorded, but controlled studies of psychological side effects resulting from this group of medications are lacking.[10-13]
This report includes a component of a multicenter study of asthma treatment conducted by the American Academy of Allergy and Immunology. In that study, the relative effectiveness of beclomethasone dipropionate was compared with that of theophylline in improving asthma symptoms, sparing supplemental bronchodilator and systemic steroid use, preventing absence from school or work, and avoiding side effects such as headaches, insomnia, gastrointestinal distress, and impeded growth velocity.[14] A battery of psychological tests also was administered to participants in each of the two blinded treatment groups at baseline, 1 month, and 1 year after treatment onset. These results have not been described previously but are presented here to provide the first data examining the long-term (1-year) psychological effects in asthmatic children treated with theophylline or inhaled corticosteroid.
CONCLUSIONS
This study represents the most comprehensive investigation conducted to date with a pediatric sample of sufficient size to detect psychological side effects of asthma medications. The preponderance of evidence, which includes scores from a large battery of standardized psychological measures and parental observations, indicates that neither inhaled beclomethasone nor theophylline causes major changes in behavior or cognition. The two significant treatment-by-period interactions appear isolated and discordant, with one suggesting slightly better attention in the theophylline group, whereas the other indicates a small advantage in attention scores in the beclomethasone group. The numerous significant period effects consistently reveal improvement in behavior or cognitive performance over the 1-year period, regardless of treatment. This finding confirms a well established practice effect resulting from repeated administrations of cognitive tests and the tendency for parents to rate their children’s behavior as less problematic over time.[15] Whatever its source, score improvement over the 1-year period is incompatible with any detrimental effect from theophylline or beclomethasone. Neither medication was found to interfere with attention, memory, discrimination, problem-solving, academic achievement, intelligence level, or emotional or behavioral adjustment.
Paper: A Comparison of Low-Dose Inhaled Budesonide plus Theophylline and High-Dose Inhaled Budesonide for Moderate Asthma
Authors: Evans D, Taylor D, Zetterstrom O, Chung K et al
Ref: N Engl J Med 1997; 337: 1412-8
Summary: Inhaled glucocorticoids are the mainstay of treatment in patients with moderate asthma. The dose used is usually titrated against symptoms. They are a relatively expensive medication particularly valued for their anti-inflammatory effects on the lungs. Theophylline is an oral bronchodilator used in asthma for over 50 years. Its use is decreasing probably due to a perceived lack of anti-inflammatory effect (although new evidence is challenging this assumption) and fears over toxicity associated with high plasma levels. It is, however, extremely cheap. Recent National Asthma Education and Prevention Program (NAEPP) guidelines advise the addition of theophylline (or long-acting beta-agonist) to medium dose inhaled glucocorticoids before initiating the long-term use of high-dose inhaled glucocorticoids. This randomized, double-blind controlled trial from Britain and Sweden investigates the effectiveness of such a strategy.
All patients recruited had typical symptoms of asthma and fulfilled the American Thoracic Society criteria for asthma. Despite treatment with inhaled budesonide 800-1000 mcg daily (or other inhaled glucocorticoid at equivalent dose) patients had continuing asthma symptoms. Forced expiratory volume in one second (FEV1) was greater then 50% predicted and improved by at least 15% following albuterol inhalation. Following a two-week run-in period, patients were randomized to receive theophylline with lower dose inhaled budesonide or high dose budesonide alone. The theophylline group received twice daily oral theophylline 250 mg (or 375 mg if over 80 kg) and 400 mcg inhaled budesonide. The high dose budesonide group received 800 mcg inhaled budesonide twice daily with a placebo tablet matched to the theophylline.
Study treatment was given for three months. Patients were monitored every three weeks and one-week after treatment ended. Lung function tests were performed at each visit and theophylline levels measured. After 12 weeks blood cortisol levels were also performed. Diary cards were completed daily. Peak expiratory flow (PEF), symptoms, and use of rescue albuterol medication were recorded.
Sixty-six patients underwent randomization, 31 in each group completed the study. Mean age was 39 years. Compliance with study treatment was high. Both treatments resulted in improvements in lung function that were sustained throughout the trial. In the high dose budesonide group the FEV1 had increased from 2.50 L to 2.62 L by 9 weeks, in the theophylline group the increase was from 2.48 L to 2.76 L; only the latter increase was statistically significant. PEF measurements increased by the end of the trial significantly in both groups: from 411 to 436 L/min in the high dose budesonide group and from 430 to 464 L/min in the theophylline group. Overall changes in PEF were statistically similar in the two groups, the FEV1 improved more in the theophylline group.
There were significant and similar reductions in albuterol use and home recorded variability in peak flow in both groups. There were small and similar reductions in symptom scores in both groups, it is not clear how
clinically important these were. Serum cortisol levels fell significantly from 18.4 mcg/dl to 15.9 mcg/dl in the high dose budesonide group but were unchanged in the theophylline group; the clinical significance of this difference is again uncertain. The median serum theophylline concentration in the theophylline group was 8.7 mcg/ml, which is lower than the usual therapeutic range of 10-20 mcg/dl. There were no discontinuations due to treatment side effects.
The clinical benefits to patients achieved with either regime in this study were arguably small. The median daytime daily use of albuterol fell from around two puffs to around one puff in both groups. Perhaps the patients’ asthma was already too well controlled on study entry to require a significant increase in therapy. The main finding is that there were virtually no significant differences between the two treatment regimens in any of the outcome variables measured. Long-term outcomes were not recorded, over time differing anti-inflammatory effects may produce more of a divergence. These results suggest that the addition of low-dose theophylline to inhaled glucocorticoid is at least as good as increasing the dose of inhaled glucocorticoid. It can be done without significant risk of toxicity occurring and is very much cheaper.
Is there a role for theophylline in treating patients with asthma? - Clinical Inquiries: from the Family Practice Inquiries Network
Journal of Family Practice, Sept, 2002 by Charissa Fotinos, Sherry Dodson
EVIDENCE-BASED ANSWER with adults, oral theophylline may help lower the dosage of inhaled steroids needed to control chronic asthma. It offers no benefit for acute asthma exacerbations. For children, intravenous aminophylline may improve the clinical course of severe asthma attacks. Side effects and toxicity limit use of these medications in most settings. (Grade of recommendation: A, based on systematic reviews and randomized control trials [RCTs]).
EVIDENCE SUMMARY Several systematic reviews help clarify theophylline’s role in asthma management. When compared with placebo in the management of acute exacerbations, theophylline confers no added benefit to beta-agonist therapy (with or without steroids) in improving pulmonary function or reducing hospitalization rates. Side effects occurred more often in the theophylline group: palpitations/arrhythmias (OR = 2.9; 95% CI: 1.5 to 5.7) and vomiting (OR = 4.2; 95% CI: 2.4 to 7.4). (1) For moderately severe asthma in patients already receiving inhaled corticosteroids (ICS), theophylline as maintenance therapy equaled long-acting beta2-agonists in increasing FE[V.sub.1] and PEFR, but was less effective in controlling night time symptoms. Use of long-acting beta-agonists resulted in fewer side effects (RR = 0.38; 95%CI: 0.25-0.57). (2) When added to low-dose ICS for maintenance, theophylline was as effective as high-dose ICS alone in improving FEV1 decreasing day and night symptoms, and reducing the need for rescue medications and the incidence of attacks. This suggests theophylline has utility as a steroid sparing agent. (3)
Intravenous aminophylline does appear to be clinically beneficial for children with severe exacerbations, defined as an FEV1 of 35%-40% of predicted value. Critically ill children receiving aminophylline in addition to usual care exhibited an improved FEV1 at 24 hours (mean difference = 8.4%; 95% CI: 0.82 to 15.92) and reduced symptom scores at 6 hours: (4) The largest RCT of aminophylline in children demonstrated a reduced intubation rate (NNT = 14 CI: 7.8-77). (5) Children receiving aminophylline experienced more vomiting (RR = 3.69; 95%CI: 2.15-6.33). Treatment with aminophylline did not reduce length of hospital stay or the number of rescue nebulizers needed (Table). (4)
RECOMMENDATIONS FROM OTHERS Three evidence-supported guidelines concur that theophylline has a limited role as maintenance therapy for moderate-to-severe persistent asthma when symptom control with ICS alone is not adequate. Much stronger evidence supports the use of long-acting beta2-agonists or leukotriene modifiers in this setting. (6-8) The guidelines do not recommend using theophylline to treat acute asthma exacerbations; nor do they address using theophylline in children.
REFERENCES
1. Wilson AJ, Gibson, PG, Coughlan J. The Cochrane Library, Issue 2, 2002 Oxford: Update Software.
2. Parameswaran K, Belda J, Rowe BH. The Cochrane Library, Issue 2. 2002, Oxford: Update Software.
3. Evans DJ, Taylor DA, Zetterstrom O, et al. N Engl J Med. 1997; 337:1412-8.
4. Mitra A, Bassler D, Ducharme FM. The Cochrane Library, Issue 2, 2002, Oxford: Update Software.
5. Yung M, South M. Arch Dis Child 1998;79: 405-410.
6. Management of Chronic Asthma. Evidence Report/Technology Assessment Number 44. AHQR Publication Number 01-E043, September 2001.
7. Global Initiative for Asthma, National Heart, Lung and Blood Institute, (U.S.)/World Health Organization 1995 Jan (revised 1998).
8. Expert Panel Report 2:Guidelines for the diagnosis and management of asthma. National Asthma Education and Prevention Program/National Heart, Lung and Blood Institute (U.S.), 1997 Jul, (reprinted 1998 Apr, 1999 Mar).
Tradename Azmacort
Manufacturer Rhone-Poulenc Rorer
Treatment Class Respiratory
Indication Asthma
Mar. 16, 1998: Triamcinolone aqueous nasal spray has no measurable effect on adrenocortical function in pediatric patients with allergic rhinitis, even at the highest recommended doses (440 mcg/day), report authors in the Journal of Allergy and Clinical Immunology (1998; 101: 157-62). The small amounts of drug absorbed were shown in pharmacokinetic studies to decline rapidly, with little or no drug accumulation.
Some 80 children ages 6-12 years received placebo or one of two doses of triamcinolone (220 or 440 mcg/day) for six weeks. No effects on adrenocortical function were reflected in plasma cortisol concentrations following a cosyntropin stimulation test, nor did the drug accumulate during six hours of testing following the final triamcinolone dose after the six weeks of therapy.
Reprints: A. S. Nayak, 130 Franklin Ave., Normal, IL 61761.
The effects of intranasal triamcinolone acetonide and intranasal fluticasone propionate on short-term bone growth and hypothalamic-pituitary-adrenal axis in children with allergic rhinitis
Pediatrics, August, 2004 by Sally Joo BaileySkoner DP, Gentile D, Banerji D, et al. Ann Allergy Asthma Immunol. 2003;90:56-64
Purpose of the Study. To evaluate the effects of triamcinolone acetonide (TAA) and fluticasone propionate (FP) aqueous nasal sprays on short-term, lower-leg growth velocity and hypothalamic-pituitary-adrenal (HPA) axis function among pediatric subjects.
Study Population. The subjects were 59 children (4-10.5 years of age) who were within normal limits for height and had a [greater than or equal to] 1-year history of allergic rhinitis that required treatment and positive prick skin test responses to an inhalant allergen. Patients who had used corticosteroids in the previous 60 days were excluded from the study.
Methods. The study was a randomized, 4-way, crossover trial comparing 2 doses of TAA nasal spray, 1 dose of FP nasal spray, and placebo among pediatric patients with perennial allergic rhinitis. The study was conducted from October 1998 through September 1999, at Children’s Hospital of Pittsburgh (Pittsburgh, PA). After a 2-week baseline period, subjects entered 4 treatment periods, each lasting 2 weeks, with a 2-week washout period between treatments. Lower-leg growth velocity was measured knemometrically. HPA axis function was assessed by measuring 12-hour (overnight) urine samples for cortisol/creatinine ratios. Three clinic visits occurred during each treatment period.
Results. Of the 59 subjects, 49 completed the study in all 4 treatment periods. Four subjects discontinued participation because of adverse events (110-[micro]g TAA group: broken foot, nasal burning sensation, asthma exacerbation; placebo group: asthma exacerbation), 3 were lost to follow-up monitoring, 1 withdrew consent, and 2 were noncompliant. In terms of lower-leg growth velocity, no differences were found between either dose of TAA and FP or between the treatment group and the placebo group. In terms of HPA axis function, the urinary cortisol/creatinine ratios from the beginning to the end of the 2-week treatment period did not differ significantly between the TAA doses and placebo; however, the mean value for the FP group was lower than those seen for other treatment groups (statistically significant). Because the coefficient of variation for the cortisol measurements was quite high, the clinical relevance of this finding is unclear.
Conclusions. This study showed that daily use of nasal sprays with TAA at 110 [micro]g, TAA at 220 [micro]g, or FP at 200 [micro]g did not produce any clinically meaningful effects on lower-leg growth velocity during the 2 weeks of treatment. FP was shown to produce a statistically significant level of HPA axis suppression, compared with placebo; however, the clinical relevance of this finding is unclear.
Reviewer’s Comments. Many pediatricians and parents have concerns regarding the effects of corticosteroid use, whether for treatment of allergic rhinitis (as a nasal spray) or for treatment of asthma, on the growth and HPA axis function of children. This study provides additional reassurance that short-term use of nasal corticosteroid sprays at standard doses does not affect growth or the HPA axis.
Sally JOO BAILEY, MD
Generic Name NA
Manufacturer AstraZeneca Pharmaceuticals
Treatment Class Respiratory
Indication Reduce the symptoms of breathlessness, cough, and sputum production that plague sufferers of chronic obstructive pulmonary disease (investigational; not approved in US)
by Rosemarie Foster, BA, MA
|
Viozan |
|
|
Brand Name: |
Viozan |
|
Active Ingredient: |
NA |
|
Indication: |
Reduce the symptoms of breathlessness, cough, and sputum production that plague sufferers of chronic obstructive pulmonary disease (investigational; not approved in US) |
|
Company Name: |
AstraZeneca Pharmaceuticals |
|
Availability: |
investigational; not approved in US |
Introduction
Patients with chronic obstructive pulmonary disease (COPD, including chronic
bronchitis and emphysema) have relied on drugs such as inhaled salbutamol and
ipratropium bromide, oral theophylline, and corticosteroids to deal with the
muscle spasms, inflammation, and increased secretions associated with their
disease. But for many patients, these agents do not do enough.
A new alternative is currently under investigation. Several presentations made at the American Thoracic Society annual meeting in Toronto in May supported the efficacy of the drug Viozan (AR-C68397AA) for reducing the symptoms of breathlessness, cough, and sputum production that plague COPD sufferers. The drug is delivered via a pressurized metered dose inhaler and is manufactured by AstraZeneca. AstraZeneca anticipates filing a new drug application in the US by the latter part of 2001.
How It Works
Viozan acts as a dual D2 dopamine
receptor agonist and a beta2-adrenoreceptor agonist. It was designed
to inhibit sensory nerve activity in the lung and produces bronchodilation.
Clinical Study
Results
Two phase II randomized,
double-blind, placebo-controlled studies have been completed to evaluate the
efficacy of Viozan. In the first study, the efficacy and tolerability of Viozan
were compared to that of placebo as well as salbutamol and ipratropium bromide.
In a 4-week study, 701 COPD patients were randomized to receive one of three
Viozan doses (400 mcg, 600 mcg, or 1000 mcg), salbutamol (200 mcg), ipratropium
bromide (40 mcg), or placebo 3 times daily. The 600 mcg dose of Viozan produced
a significant reduction in COPD symptoms compared to placebo. At the dose
regimens tested, salbutamol and ipratropium bromide showed only limited
efficacy.
In the second study, the efficacy and tolerability of Viozan were investigated in a 6-week trial of 872 COPD patients who were randomized to receive one of three Viozan doses (45 mcg, 270 mcg, or 495 mcg) or placebo 3 times daily. Viozan reduced COPD symptoms in a dose-dependent manner, these differences being statistically significant with the two higher doses. At the two higher doses the improvement in symptoms was accompanied by a significant reduction in use of rescue bronchodilator as well as increases in patients’ health-related quality of life as measured using the St. Georges Respiratory Questionnaire.
Adverse Events
Safety and tolerability data
indicated that Viozan was generally well tolerated. Adverse events that were
more common in the Viozan group compared to placebo included reported in the
groups studied included tremor, nausea, and taste of treatment.
1. Laitinen, LA, et al. “Efficacy of Viozan (AR-C68397AA) versus salbutamol and ipratropium bromide in the management of COPD.” Presented at the 96th meeting of the American Thoracic Society, May 2000.
2. Wenzel, S, et al. “Viozan (AR-C68397AA) reduces breathlessness, cough and sputum production in COPD patients.” Presented at the 96th meeting of the American Thoracic Society, May 2000.
3. Wenzel, S, et al. “Viozan (AR-C68397AA) improves health-related quality of life in patients with COPD.” Presented at the 96th meeting of the American Thoracic Society, May 2000.
4. Ind, PW, et al. “Tolerability profile of Viozan (AR-C68397AA): A novel therapy for symptomatic treatment of COPD.” Presented at the 96th meeting of the American Thoracic Society, May 2000.
5. Merck Manual, Home Edition, 1997 edition, page 179.
Tradename Accolate
Manufacturer AstraZeneca
Treatment Class Respiratory
Indication Asthma
August, 1995: Special to the Medical Sciences Bulletin
A variety of novel approaches are being investigated to control the symptoms of asthma. One interesting approach is the administration of drugs that block the inflammatory component of asthma. Leukotrienes have been known to play a role in the inflammatory cascade and one new agent designed to block the activity of leukotrienes, Accolate, has shown significant clinical promise. The following research results were presented at a recent scientific meeting and highlight the activity of this therapeutic agent.
Two New Studies
Provide Further Evidence for Effectiveness of Accolate
The results of two new studies on the
efficacy and safety of the leukotriene-receptor antagonist Accolate (tm)
(also known as ICI 204,219) were presented at the recent annual meeting of the
American Academy of Allergy and Immunology in New York.
Each study was 13 weeks in length, multicenter, double-blind, and involved patients with self-assessed mild-to-moderate asthma. The first study (Lockey RF et al, Abstract), which involved 762 patients compared the effects of Accolate with those of placebo. Patients treated with Accolate had improvements compared to placebo in daytime asthma symptom scores (p less than 0.01), nighttime awakenings (p less than 0.05), mornings with asthma (p less than 0.01) beta-agonist use (p less than 0.01) and morning PEFR (+14 L/min, p less than 0.01). FEV1 percent predicted increased to >80% in the active treatment group, which was significant (p less than 0.01) compared to placebo.
In the second study, (Nathan RA et al, Abstract) 287 patients with mild to moderate asthma were randomized to receive Accolate (20 mg bid), cromolyn sodium (2 puffs qid) or placebo. More patients responded to treatment with Accolate (64%) and cromolyn (68%) than with placebo (46%, p less than 0.05); no difference was observed between the active treatment groups. Treatments were as well tolerated as placebo.
Accolate is an oral tablet product being developed by Zeneca for first-line chronic prophylaxis and treatment of adult and adolescent asthma. The compound is a highly selective and potent antagonist of leukotrienes. These compounds are important mediators of inflammatory bronchospastic diseases and blocking their activity results in the control of inflammatory symptoms of asthma. Accolate is currently undergoing Phase III investigations.
From the November 1996 issue [Med Sci Bull. 1996;19(3):1] of Medical Sciences Bulletin
The FDA has approved the leukotriene receptor antagonist zafirlukast (Accolate/Zeneca), the first truly new asthma drug to hit the market in 25 years. Zafirlukast blocks receptors for the cysteinyl leukotrienes C4, D4, and E4 (that is, leukotrienes bound to the amino acid cysteine). The cysteinyl leukotrienes are potent bronchoconstrictors, approximately 100 to 1,000 times more potent than histamine. By blocking receptors that mediate bronchoconstriction, vascular permeability, and mucous secretion, zafirlukast significantly improves the wheezing, coughing, and dyspneic symptoms of asthma. The drug is indicated for chronic asthma therapy; it is not a bronchodilator and should not be used to reverse bronchospasm, although therapy can be continued during acute attacks.
In randomized double-blind clinical trials involving 1,380 patients with mild-to-moderate asthma, zafirlukast improved daytime asthma symptoms, nighttime awakenings, mornings with asthma symptoms, rescue beta2-agonist use, forced expiratory volume, and morning peak expiratory flow rate. Safety trials involving more than 4,000 patients, some of whom took zafirlukast for more than one year, indicate that zafirlukast is generally safe and well tolerated.
The most common side effect reported in clinical trials was headache, which occurred in 12.9% of zafirlukast patients and 11.7% of placebo patients. Infection, nausea, and diarrhea were reported by approximately 3% of zafirlukast patients and 2% of placebo patients. All other side effects were reported with the same frequency (less than 2%) by both groups.
Zafirlukast is rapidly absorbed orally; peak plasma concentrations are achieved at three hours after dosing. Administration with food reduces mean bioavailability by about 40%. Zafirlukast is 99% protein bound and is extensively metabolized in the liver (cytochrome P450-2C9 system); 10% of the dose is excreted in the urine and the rest in the feces. Mean terminal elimination half-life is approximately 10 hours. Clearance is reduced in the elderly and in patients with hepatic impairment. Because zafirlukast is metabolized by the P450 system, there is the potential for interaction with other drugs metabolized by the same system. Studies conducted so far show that zafirlukast prolongs the half-life of warfarin, and that zafirlukast pharmacokinetics are altered by concomitant administration of terfenadine, erythromycin, theophylline, and aspirin.
Precautions
Zafirlukast should not be used in
pregnancy (unless clearly needed) and should not be used during breast feeding.
Safety and efficacy have not been established in children younger than age 10.
Available in 20-mg tablets, zafirlukast is administered 20 mg twice daily on an
empty stomach (one hour before or two hours after meals). (Holgate ST
et al. J Allergy Clin Immunol.
1996;98:1-13. Spector SL. Annals of Allergy, Asthma, Immunol. 1995;75:473-474.
Spector SL et al. Am J Respir Crit Care Med 1994;150:618-623. Additional
information from the manufacturer.)
Fluticasone vs zafirlukast in asthma treatment
Summary
Several controlled clinical trial studies have shown the overall superiority of
fluticasone (Flut), an inhaled corticosteroid, over zafirlukast (Zaf) a
leukotriene antagonist (LA) drug in the treatment of asthma. However, it has
been uncertain whether such superiority of Flut occurs in community clinical
practice. Therefore, Stempel et al of the Virginia Mason Clinic in Seattle, WA
carried out a prospective comparison of the clinical course of about 700
asthmatic patients started on either Flut or Zaf, monitoring that these drugs
had actually been used. Over a year’s follow-up period there had been 70% fewer
hospitalizations and 49% less ER use by the Flut treated asthmatics than the
Zaf treated patients. Overall asthma care costs were reduced in the
Flut-treated group and increased in the Zaf- treated group. The authors
concluded that experience in clinical practice confirmed the superiority of
Flut over Zaf in asthma treatment.
Reference
J
Allergy Clin Immunol 2001;107:94-98
Adrenergic drugs used to treat patients with pulmonary problems can be divided into three classes:
1. Catecholamines: a group of compounds having a sympathomimetic action, the aromatic portion of whose molecule is catechol and the diapnotic portion. Examples of catecholamines are dopamine, epinephrine, isoproterenol, norepinephrine, and isoetharine.
2. Resorcinols: modification of the catechol nucleus produces a resorcinol which is not metabolized by the enzyme COMT, giving them a longer duration of action, while the size of the amine side chain confers beta2 specificity for metaproterenol and terbutaline.
3. Saligenins: a different modification of the catechol nucleus results in the saligenin albuterol. Pirbuterol is structural similar to albuterol, is less potent on a weight basis, but is similar in both efficacy and toxicity to metaproterenol.
Epinephrine (Adrenalin, Bronkaid, Primatene) is often held up as the standard against which other sympathomimetics are judged. It is a powerful bronchodilator and decongestant that can be administered by injection or by aerosol, and an agent that causes an increase in the rate and force of heart contractions, dilation of the bronchial smooth muscles, and constriction of the peripheral vasculature. It is used parenterally for the relief of acute bronchial asthma. In the hospital setting, epinephrine is used frequently for its effect on the circulation, during cardiopulmonary resuscitation (CPR), and to combat allergic reactions.
Epinephrine (ep-ih-NEF-rin)
Pregnancy Category: C Adrenalin Chloride Solution Ana-Guard Bronkaid
Mistometer![]() Epi E-Z Pen Epi E-Z Pen Jr. Epipen Epipen Jr.
Primatene Mist Solution Sus-Phrine (Both Rx and OTC)
Epi E-Z Pen Epi E-Z Pen Jr. Epipen Epipen Jr.
Primatene Mist Solution Sus-Phrine (Both Rx and OTC)
Epinephrine bitartrate (Primatene Mist Suspension)
Epinephrine
(ep-ih-NEF-rin)
Pregnancy Category: C AsthmaHaler Mist (OTC)
Epinephrine borate
Epinephrine borate
Epinephrine
(ep-ih-NEF-rin)
Pregnancy Category: C Epinal Ophthalmic Solution (Rx)
Epinephrine hydrochloride
Epinephrine hydrochloride (Adrenalin Chloride)
Epinephrine
(ep-ih-NEF-rin)
Pregnancy Category: C Adrenalin Chloride AsthmaNefrin Epifrin Glaucon microNefrin Nephron S-2 Inhalant (OTC) (Rx)
Classification: Adrenergic agent, direct-acting
Action/Kinetics: Causes marked stimulation of alpha, beta1,
and beta2 receptors, causing sympathomimetic stimulation, pressor
effects, cardiac stimulation, bronchodilation, and decongestion. It crosses the
placenta but not the blood-brain barrier. Extreme caution must be taken
never to inject 1:100 solution intended for inhalation—injection of this
concentration has caused death. SC: Onset, 5-10 min; duration: 4-6
hr. Inhalation: Onset 1-5 min; duration: 1-3 hr. IM, Onset: variable;
duration: 1-4 hr. Ineffective when given PO.
Uses: Inhalation: Temporary relief of shortness of breath,
tightness of chest, and wheezing due to bronchial asthma. Postintubation and
infectious coup. MicroNefrin is used for chronic obstructive lung disease,
chronic bronchitis, bronchiolitis, bronchial asthma, and other peripheral
airway diseases.
Injection: Relieve respiratory distress in bronchial asthma, during
acute asthma attacks, and for reversible bronchospasm in chronic bronchitis,
emphysema, and other obstructive pulmonary diseases. Severe acute anaphylactic
reactions, including anaphylactic shock and cardiac arrest, to restore cardiac
rhythm. Allergic reactions caused by bees, wasps, hornets, yellow jackets,
bumble bees, and fire ants; severe allergic reactions or anaphylaxis caused by
allergy injections; allergic reactions due to exposure to pollens, dusts,
molds, foods, drugs, and exercise. Severe, life-threatening asthma attacks with
wheezing, dyspnea, and inability to breathe. Vasopressor in shock. Infiltration
of tissue to delay absorption of drugs, including local anesthetics.
NOTE: Autoinjectors are available for emergency self-administration of
first aid for anaphylactic reactions due to insect stings or bites, foods,
drugs, and other allergens as well as idiopathic or exercise-induced
anaphylaxis.
Special Concerns: May cause anoxia in the fetus. Safety and efficacy of ophthalmic products have not been determined in children; administer parenteral epinephrine to children with caution. Syncope may occur if epinephrine is given to asthmatic children. Administration of the SC injection by the IV route may cause severe or fatal hypertension or cerebrovascular hemorrhage. Epinephrine may temporarily increase the rigidity and tremor of Parkinsonism. Use with caution and in small quantities in the toes, fingers, nose, ears, and genitals or in the presence of peripheral vascular disease as vasoconstriction-induced tissue sloughing may occur. May temporarily increase rigidity and tremor in Parkinson’s disease.
Additional Side Effects: CV: Fatal ventricular fibrillation, cerebral or subarachnoid hemorrhage
obstruction of central retinal artery. A rapid and large increase in BP may cause aortic rupture, cerebral
hemorrhage, or angina pectoris. GU: Decreased urine
formation, urinary retention, painful urination. CNS: Anxiety, fear,
pallor. Parenteral use may cause or aggravate disorientation, memory
impairment, psychomotor agitation, panic, hallucinations, suicidal or homicidal tendencies
schizophrenic-type behavior. Miscellaneous: Prolonged use or overdose
may cause elevated serum lactic acid with severe metabolic acidosis. At
injection site: Bleeding, urticaria, wheal formation, pain. Repeated
injections at the same site may cause necrosis from vascular constriction. Ophthalmic:
Transient stinging or burning when administered, conjunctival hyperemia, brow
ache, headache, blurred vision, photophobia, allergic lid reaction, ocular
hypersensitivity, poor night vision, eye ache, eye pain. Prolonged ophthalmic
use may cause deposits of pigment in the cornea, lids, or conjunctiva. When
used for glaucoma in aphakic clients, reversible cystoid macular edema.
Laboratory Test Alterations: False + or ![]() BUN, fasting glucose, lactic acid, urinary
catecholamines, glucose (Benedict’s).
BUN, fasting glucose, lactic acid, urinary
catecholamines, glucose (Benedict’s). ![]() Coagulation time. The drug may affect electrolyte
balance.
Coagulation time. The drug may affect electrolyte
balance.
Additional Drug Interactions: Alpha-adrenergic blocking agents /
Antagonism of vasoconstricting and hypertensive effects Antihistamines /
Epinephrine effects potentiated Beta-adrenergic blocking agents /
Possible initial hypertension followed by bradycardia Diuretics / ![]() Vascular
response Ergot alkaloids / Reversal of epinephrine pressor effects General
anesthetics (halothane, cyclopropane) /
Vascular
response Ergot alkaloids / Reversal of epinephrine pressor effects General
anesthetics (halothane, cyclopropane) / ![]() Sensitivity
of myocardium to epinephrine
Sensitivity
of myocardium to epinephrine ![]() arrhythmias Levothyroxine / Potentiation of
epinephrine effects Nitrites / Reversal of epinephrine pressor effects Phenothiazine
/ Reversal of epinephrine pressor effects
arrhythmias Levothyroxine / Potentiation of
epinephrine effects Nitrites / Reversal of epinephrine pressor effects Phenothiazine
/ Reversal of epinephrine pressor effects
How Supplied: Epinephrine: Aerosol: 0.2 mg/inh; Injection:
1:200 (5 mg/mL), 1:1000 (1 mg/mL), 1:10,000 (0.1 mg/mL); Kit: 0.5 mg/mL,
1 mg/mL; Solution for Inhalation (Racepinephrine HCl): 2.25%.
Epinephrine bitartrate: Metered dose inhaler: 0.35 mg/inh. Epinephrine
hydrochloride: Solution for Inhalation: 1:100, 1:1000. Ophthalmic
solution: 0.5%, 1%, 2%
Dosage
·
Nebulization Bronchodilation.
Adults and children 4 years and older (for AsthmaNefrin, 12 years and
older). Hand pump nebulizer: 0.5 mL (about 8-10 drops) of racemic
epinephrine placed into the reservoir. Place the nebulizer nozzle into the
partially opened mouth and squeeze the bulb 1-3 times. Inhale deeply. Give 2-3
additional inhalations if relief does not occur within 2-3 min. Can use 4-6
times/day but no more often than q 3 hr. Aerosol nebulizer: Add 0.5 mL
(about 10 drops) of racemic epinephrine to 3 mL of diluent or 0.2-4 mL (about
4-8 drops) of MicroNefrin to 4.6-4.8 mL water. Give for 15 min q 3-4 hr.
·
SC, IM Bronchodilation
Adults, initial: 0.2-1 mL (0.2-1 mg) of the 1:1000 solution SC or IM q 4
hr. Infants and children (except premature infants and full-term newborns):
0.01 mL/kg or 0.3 mL/m2 (0.1 mg/kg or 0.3 mg/m2) SC. Do
not exceed 0.5 mL (0.5 mg) in a single pediatric dose. Can repeat q 20 min to 4
hr, if necessary.
The dose of Ana-Guard is as follows. Adults and children over 12 years:
0.3 mL; 6-12 years old: 0.2 mL; 2-6 years old: 0.15 mL; infants
to 2 years old: 0.05-0.1 mL. Give a second dose after 10 min if symptoms
are not noticeably improved.
·
IV Bronchodilation, hypersensitivity reactions.
Adults: 0.1-0.25 mg (1-2.5 mL) of the 1:10,000 solution injected slowly.
Infants: 0.05 mg; may be repeated at 20-30 min intervals to manage
asthma attacks. Neonates: 0.01 mg/kg.
Note: If the client is intubated, the IV dose of epinephrine can be given
via the endotracheal tube directly into the bronchial tree as it is rapidly
absorbed through the lung capillary bed.
·
SC only, 1:200 Suspension Bronchodilation.
Adults: 0.1-0.3 mL (0.5-1.5 mg). Children, less than 30 kg:
Maximum single dose is 0.15 mL (0.75 mg). Infants and children, 1 month to
12 years old: 0.005 mg/kg (0.025 mg/kg).
·
Autoinjector, IM First aid for anaphylaxis.
The autoinjectors deliver a single dose of either 0.3 mg or 0.15 mg (for
children) of epinephrine. In cases of a severe reaction, repeat injections may
be necessary.
·
Vasopressor.
Adults, IM or SC, initial: 0.5 mg repeated q 5 min if needed; then, give
0.025-0.050 mg IV q 5-15 min as needed. Adults, IV, initial: 0.1-0.25 mg
given slowly. May be repeated q 5-15 min as needed. Or, use IV infusion
beginning with 0.001 mg/min and increasing the dose to 0.004 mg/min if needed. Pediatric,
IM, SC: 0.01
mg/kg, up to a maximum of 0.3 mg repeated q 5 min if needed. Pediatric, IV: 0.01
mg/kg/5-15 min if an inadequate response to IM or SC administration is
observed.
·
Cardiac stimulant.
Adults, intracardiac or IV: 0.1-1 mg repeated q 5 min if needed. Pediatric,
intracardiac or IV: 0.005-0.01 mg/kg (0.15-0.3 mg/m2) repeated q
5 min if needed; this may be followed by IV infusion beginning at 0.0001
mg/kg/min and increased in increments of 0.0001 mg/kg/min up to a maximum of
0.0015 mg/kg/min.
Adjunct to local anesthesia.
Adults and children: 0.1-0.2 mg in a 1:200,000-1:20,000 solution.
Adjunct with intraspinal anesthetics.
Adults: 0.2-0.4 mg added to the anesthetic spinal fluid.
·
Solution Antihemorrhagic, mydriatic.
Adults and children, intracameral or subconjunctival: 0.01%-0.1%
solution.
Topical antihemorrhagic.
Adults and children: 0.002%-0.1% solution.
Nasal decongestant.
Adults and children over 6 years of age: Apply 0.1% solution as drops or
spray or with a sterile swab as needed.
Ephedrine (Tedral, Marax, Primatene, Quadrinal) is an alkaloid derived from an herb by the Chinese over 5,000 years ago. It demonstrates similar to the action as epinephrine, but it isn’t used regularly for bronchodilation. It is one of the few Beta adrenergic agonists effective when given orally. It is considered long-acting (four to six hours) and slightly less potent than epinephrine. Tablets are the usual method of administration.
Ephedrine is a potent CNS stimulant so a tranquilizer is often added. Tachyphylaxis readily develops with ephedrine, along with excessive CNS stimulation. Excessive use by patients can cause marked mental excitation and elevation of blood pressure. Dosage varies depending upon the patient’s condition. Proprietary preparations contain between 12‑25 mg of ephedrine with 50‑130 mg of theophylline.
Isoproterenol (Isuprel) a powerful beta stimulator, both beta1 and beta2, is somewhat better bronchodilator than epinephrine, with nearly complete absence of vasopressor activity. However, its strong beta1 effects increase the probability of tachycardia, thereby limiting the frequency of its use. It is available in aqueous concentrations of 1:100 and 1:200, with the latter being the strength recommended for aerosol therapy. For short term therapy, two to four inhalations at four hour intervals is normal. Long term therapy mandates further dilution of the concentrate.
Metaproterenol (Metaprel, Alupent) a derivative of isoproterenol, it has significantly longer duration of action, and has beta1 effects when given by inhalation. Metaproterenol can be taken orally or inhaled, but is contraindicated in patients with pre-existing cardiac arrhythmias, and used extremely cautiously in patients with hypertension, coronary artery disease, congestive heart failure, hyperthyroidism, and diabetes.
Isoetharine (Bronkosol, Bronkometer) has a strong Beta2 effect with minimal Beta1 stimulation. Both of these effects are less than those of isoproterenol. Isoetharine is administered orally or by MDI, SVN, IPPB. it is reportedly more effective in conditions characterized by diffuse bronchospasm, as in bronchial asthma, than in those with obstructing secretions, as in chronic bronchitis. Duration of action is 2 to 4 hours. The usual aerosol dose of 0.5 cc of the 1% solution in 2‑3 cc NS Q4 hours may be increased to 1 cc for severe bronchospasm.
Terbutaline (Bricanyl, Brethine, Brethaire) acts directly on beta2 receptors, has minimal beta1 effects, and is more potent and long-acting than metaproterenol. Inhaled terbutaline has been shown to increase mucociliary clearance by as much as 50%. It is also available for oral and subcutaneous administration.
Albuterol (Proventil, Ventolin) is one of the most commonly used aerosolized beta2 adrenergic bronchodilators, but is also effective orally or intravenously. The aerosolized form causes fewer side effects, and it is long acting because it is not metabolized by COMT. The normal dosage when delivered by a small volume nebulizer is 2.5 mg QID. A sustained released form of albuterol is available as either Proventil Retabs or Volmax, and the extended activity lasts up to 12 hours.
Pirbuterol (Maxair) is a selective beta2 adrenergic bronchodilator similar to albuterol, and is long‑acting with a duration of action of about 5 hours. It is effective when aerosolized or given orally. Effective onset of action occurs within 15 minutes, with peak effectiveness being realized within an hour. Recommended dose for adults and children older than 12 is 0.4 mg, inhaled 2 or 3 times every 4‑6 hours.
Fenoterol is one of the newer beta adrenergic bronchodilators (although it has been used in Europe for some time). Fenoterol is available for oral or inhalation administration, and appears to have a very long duration of action (8 hours), with minimal Beta1 impact. Fenoterol has the advantage of acting mainly on the peripheral airways, and its bronchodilation is proportional to isoproterenol. On the other hand, it has more side effects than seen with albuterol or terbutaline, and has been associated with an increase in morbidity and mortality.
Bitolterol (Tornalate) is known as a pro-drug, meaning that the administered form must be converted in the body to become an active drug. Since the process of activation begins when the drug is administered and gradually continues over time, duration of action is prolonged to about 8 hours. It has been compared favorably with albuterol, and has fewer side effects than fenoterol with similar in duration of action. Each activation of the MDI delivers about 0.37 mg of bitolterol, and recommended dosage is 2 puffs every 8 hours with maximum dosage not to exceed 2 puffs every 4 hours.
Salmeterol Xinafoate (Serevent) received FDA approval in 1994, and represents a new generation of long-acting beta2 specific bronchodilators whose pharmakinetics provide for a slower onset and time to peak effect, and a longer duration of action than seen in other adrenergic agents. It is indicated for long-term maintenance therapy of asthma which cannot be controlled by occasional use of beta agonists, and for the prevention of bronchospasm in patients needing maintenance therapy for reversible
airway obstruction. Salmeterol is a highly selective beta2 adrenergic. It should not be initiated in patients with significantly worsening or acutely deteriorating asthma, and the recommended dosage should never be exceeded. Dosage as an MDI is 2 puffs (42mcg) Q12 hours, and its side effects and safety profile are similar to other bronchodilators.
Formoterol is a catecholamine analogue with beta2-selective action. It is similar to salmeterol in its long-acting effectiveness of about 12 hours, maintaining FEV1 values 20% above baseline in patients with stable asthma. Dosage is the same as salmeterol, simplifying administration for asthmatics.
Standard recommended dosages for bronchodilators have been established for patients with respiratory problems, but are not necessarily designed for those with severe bronchospasm or in status asthmaticus. Treatment of more severe bronchial problems require increasing both the dosage and frequency of aerosol medications. The greater the degree of the patient’s bronchial constriction:
· the greater the amount of bronchodilator needed
· the faster the bronchodilator is degraded so more frequent administration also is necessary
Many of the newer compounds have been developed to allow for stronger and faster acting bronchodilation. Response to a drug is proportional to the drug concentration. As the drug concentration increases, the number of receptors occupied continues to increase until the drug has occupied all available receptors. The rate of response to the drug usually begins to diminish as dosages increase, until a ceiling of maximal effect is reached. At this point, delivering more drug elicits no further therapeutic effect.
The term potency refers to the concentration or dose a drug producing 50% of that drug’s maximal response, and maximal response refers to the greatest response that can be produced by the drug, a dose above which no further response can be elicited. When acceptable and maximal dosages are being established for individual drugs, the ratio of the dose which provides relief to 50% of the test subjects, to the dose which is toxic or lethal to 50% of the subjects, is called the therapeutic index (TI).
This TI ratio represents a safety margin for the drug, meaning the smaller the TI, the greater the possibility of crossing from a therapeutic effect to a toxic effect. For example, theophylline is an example of a drug with a fairly narrow therapeutic margin. Toxic side effects from theophylline can be seen in some individuals at dose levels that are very close to dose levels that are therapeutic for other patients.
Patients with more severe respiratory ailments benefit from the fact that many of the new bronchodilating compounds have a wider TI, allowing caregivers to safely administer maintenance doses that can be up to 8 or 10 times the conventionally recommended dose. In treating these more severe cases, caregivers test several combinations of dosage and frequency to arrive at what seems to be the optimal dosage regimen for each individual. Since patients with artificial airways actually receive far less of the drug being administered than nonintubated patients, they can also benefit from higher than normally recommended dosage regimens.
Theophylline has more than twenty different trade names, including (Aerolate, Aquaphyllin, Bronkodyl, Slo-bid Gyrocaps, Theon, Respbid, and Theolair). It is related chemically to the natural metabolite xanthine, which is a precursor of uric acid. Xanthines have a wide variety of physiologic effects on humans, ranging from CNS stimulation to bronchial smooth muscle relaxation and cerebral vasoconstriction. The effects of xanthines are well known to people who drink caffeinated beverages like coffee, colas, and tea.
Theophylline is available in liquid, tablet, and capsule form. Aminophylline delivered via aerosol has been tested for treating asthmatic patients and most of the evidence indicated that it has minimal impact on forced expiratory flow rates in patients with acute obstructive disorders. It also was found to irritate the pharynx, caused coughing and wheezing, and had a very bitter taste.
While the exact mechanism of action of xanthines, particularly theophylline, remains relatively unknown, they have had a variety of clinical uses. Traditionally, theophylline has been used in the management of asthma and COPD. While it is usually classified as a bronchodilator, theophylline is relatively ineffective when compared with the beta agonists.
Recent clinical findings have shown that its use provides no additional benefit when patients have already been treated with intravenous steroids and inhaled beta adrenergic bronchodilators. The NIH’s 1997 Guidelines for Diagnosis and Management of Asthma do not indicate theophylline as first line therapy for the pharmacologic management of asthma and COPD. However, theophylline remains very popular and effective, although its use is frequently being relegated to a secondary or tertiary role after beta agonists, inhaled steroids, or mediator agents.
Besides its use for bronchodilation, theophylline has been shown to increase patient’s minute volume by improving contractility of a normal or fatigued diaphragm. Because it stimulates the phrenic nerve, theophylline has proven useful in reducing the severity of sleep apnea in adults and apnea in neonates. However, because there is little difference between dose and serum levels that provide therapeutic benefit and those which cause toxicity (a narrow TI), theophylline has a wide variety of potential side effects throughout the body’s organ systems, including:
· Central nervous system—headache, anxiety, insomnia, restlessness, tremor, and convulsions
· Gastrointestinal system—nausea, vomiting, abdominal pain, diarrhea, hematemesis, gastroesophageal reflux, and anorexia
· Respiratory system—tachypnea
· Cardiovascular system—hypotension, palpitations, supraventricular tachycardia, ventricular arrhythmias
· Renal system—diuresis
Proper dosing of theophylline is complicated because of the variability in the rate at which individuals metabolize it. Different forms of the drug are not always equivalent, and there are a variety of factors that can affect the half-life of theophylline. This includes cigarette or marijuana smoking, high caffeine intake, barbiturate use, high protein diets, liver disease, marked obesity, severe hypoxemia, or fever. As a result, dosing schedules, which are available in product literature and references such as the Physician’s Desk Reference, are used to titrate the drug. It is recommended that these tables be consulted when administering theophylline for different ages and clinical applications.
Theophylline (thee-OFF-ih-lin)
Pregnancy Category: C Immediate-release Capsules,
Tablets: Bronkodyl Elixophyllin Quibron-T Dividose Slo-Phyllin Theolair.
Liquid Products: Accurbron Aquaphyllin Asmalix Elixomin
Elixophyllin Lanophyllin Pulmophylline![]() Quibron-T/SR
Quibron-T/SR![]() Slo-Phyllin Theoclear-80 Theolair Theolixir
Slo-Phyllin Theoclear-80 Theolair Theolixir![]() Theostat-80 Theophylline Oral. Timed-release Capsules: Slo-bid Gyrocaps
Slo-Phyllin Gyrocaps Theo-24 Theobid Duracaps Theoclear L.A.-130 Theoclear
L.A.-260 Theospan-SR Theovent.
Theostat-80 Theophylline Oral. Timed-release Capsules: Slo-bid Gyrocaps
Slo-Phyllin Gyrocaps Theo-24 Theobid Duracaps Theoclear L.A.-130 Theoclear
L.A.-260 Theospan-SR Theovent.
Timed-release Tablets: Apo-Theo LA![]() Novo-Theophyl SR
Novo-Theophyl SR![]() Quibron-T/SR Dividose Respid Sustaire Theochron Theochron-SR
Quibron-T/SR Dividose Respid Sustaire Theochron Theochron-SR![]() Theo-Dur Theolair
Theo-Dur Theolair![]() Theolair-SR Theophylline Extended-release
Theophyline SR Theo-Sav Theo-SR
Theolair-SR Theophylline Extended-release
Theophyline SR Theo-Sav Theo-SR![]() Theo-X T-Phyl Uni-Dur Uniphyl. (Rx)
Theo-X T-Phyl Uni-Dur Uniphyl. (Rx)
Classification: Antiasthmatic, bronchodilator
Action/Kinetics: Theophylline stimulates the CNS, directly relaxes the smooth muscles of the bronchi and pulmonary blood vessels (relieve bronchospasms), produces diuresis, inhibits uterine contractions, stimulates gastric acid secretion, and increases the rate and force of contraction of the heart. Directly relaxes the bronchiolar smooth muscle (relieves bronchospasm) and pulmonary blood vessels. Although the exact mechanism is not known, theophyllines may alter the calcium levels of smooth muscle, blocking adenosine receptors, inhibiting the effect of prostaglandins on smooth muscle, and inhibiting the release of slow-reacting substance of anaphylaxis and histamine. Well absorbed from uncoated plain tablets and PO liquids. Time to peak serum levels, oral solution: 1 hr; uncoated tablets: 2 hr; chewable tablets: 1-1.5 hr; enteric-coated tablets: 5 hr; extended-release capsules and tablets: 4-7 hr. Therapeutic plasma levels: 10-20 mcg/mL. t1/2: 3-15 hr in nonsmoking adults, 4-5 hr in adult heavy smokers, 1-9 hr in children, and 20-30 hr for premature neonates. An increased t1/2 may be seen in individuals with CHF, alcoholism, liver dysfunction, or respiratory infections. Because of great variations in the rate of absorption (due to dosage form, food, dose level) as well as its extremely narrow therapeutic range, theophylline therapy is best monitored by determination of the serum levels. In healthy adults, about 60% is bound to plasma protein whereas in neonates 36% is bound to plasma protein. Eighty-five percent to 90% metabolized in the liver and various metabolites, including the active 3-methylxanthine. Theophylline is metabolized partially to caffeine in the neonate. The premature neonate excretes 50% unchanged theophylline and may accumulate the caffeine metabolite. Excretion is through the kidneys (about 10% unchanged in adults).
Uses: Prophylaxis and treatment of bronchial asthma. Reversible
bronchospasms associated with chronic bronchitis, emphysema, and COPD. Oral
liquid: Neonatal apnea as a respiratory stimulant. Theophylline and
dextrose injection: Respiratory stimulant in neonatal apnea and
Cheyne-Stokes respiration.
Contraindications: Hypersensitivity to any xanthine, peptic ulcer,
seizure disorders (unless on medication), hypotension, CAD, angina pectoris.
Special Concerns: Use during lactation may result in irritability, insomnia, and fretfulness in the infant. Use with caution in premature infants due to the possible accumulation of caffeine. Xanthines are not usually tolerated by small children because of excessive CNS stimulation. Geriatric clients may manifest an increased risk of toxicity. Use with caution in the presence of gastritis, alcoholism, acute cardiac diseases, hypoxemia, severe renal and hepatic disease, severe hypertension, severe myocardial damage, hyperthyroidism, glaucoma.
Side Effects: Side effects are uncommon at serum theophylline levels
less than 20 mcg/mL. At levels greater than 20 mcg/mL, 75% of individuals
experience side effects including N&V, diarrhea, irritability, insomnia,
and headache. At levels of 35 mcg/mL or greater, individuals may manifest cardiac arrhythmias
hypotension, tachycardia, hyperglycemia, seizures, brain damage, or death. GI:
N&V, diarrhea, anorexia, epigastric pain, hematemesis, dyspepsia, rectal
irritation (following use of suppositories), rectal bleeding, gastroesophageal
reflux during sleep or while recumbent (theophylline). CNS: Headache,
insomnia, irritability, fever, dizziness, lightheadedness, vertigo, reflex
hyperexcitability, seizures
depression, speech abnormalities, alternating periods of mutism and
hyperactivity, brain damage,
death. CV: Hypotension, life-threatening ventricular arrhythmias
palpitations, tachycardia, peripheral
vascular collapse extrasystoles. Renal: Proteinuria,
excretion of erythrocytes and renal tubular cells, dehydration due to diuresis,
urinary retention (men with prostatic hypertrophy). Other: Tachypnea, respiratory arrest fever,
flushing, hyperglycemia, antidiuretic hormone syndrome, leukocytosis, rash,
alopecia.
Laboratory Test Alterations: ![]() Plasma free fatty acids, bilirubin, urinary
catecholamines, ESR. Interference with uric acid tests and tests for furosemide
and probenecid.
Plasma free fatty acids, bilirubin, urinary
catecholamines, ESR. Interference with uric acid tests and tests for furosemide
and probenecid.
Overdose Management: Symptoms: Agitation, headache, nervousness,
insomnia, tachycardia, extrasystoles, anorexia, N&V, fasciculations,
tachypnea, tonic-clonic seizures.
The first signs of toxicity may be seizures or ventricular arrhythmias.
Toxicity is usually associated with parenteral administration but can be
observed after PO administration, especially in children. Treatment:
Have ipecac syrup, gastric lavage equipment, and cathartics available to treat
overdose if the client is conscious and not having seizures. Otherwise a mechanical
ventilator, oxygen, diazepam, and IV fluids may be necessary for the treatment
of overdosage. For postseizure coma, maintain an airway and oxygenate the
client. To remove the drug, perform only gastric lavage and give the cathartic
and activated charcoal by a large-bore gastric lavage tube. Charcoal
hemoperfusion may be necessary. Treat atrial arrhythmias with verapamil and
treat ventricular arrhythmias with lidocaine or procainamide. Use IV fluids to
treat acid-base imbalance, hypotension, and dehydration. Hypotension may also
be treated with vasopressors. To treat hyperpyrexia, use a tepid water sponge
bath or a hypothermic blanket. Treat apnea with artificial respiration. Monitor
serum levels of theophylline until they fall below 20 mcg/mL as secondary rises
of theophylline may occur, especially with sustained-release products.
Drug Interactions: Allopurinol / ![]() Theophylline
levels Aminoglutethimide /
Theophylline
levels Aminoglutethimide / ![]() Theophylline levels Barbiturates /
Theophylline levels Barbiturates / ![]() Theophylline
levels Benzodiazepines / Sedative effect may be antagonized by
theophylline Beta-adrenergic agonists / Additive effects Beta-adrenergic
blocking agents /
Theophylline
levels Benzodiazepines / Sedative effect may be antagonized by
theophylline Beta-adrenergic agonists / Additive effects Beta-adrenergic
blocking agents / ![]() Theophylline levels Calcium channel blocking
drugs /
Theophylline levels Calcium channel blocking
drugs / ![]() Theophylline levels Carbamazepine / Either
Theophylline levels Carbamazepine / Either ![]() or
or ![]() theophylline
levels Charcoal /
theophylline
levels Charcoal / ![]() Theophylline levels R/T
Theophylline levels R/T ![]() metabolism
Cimetidine /
metabolism
Cimetidine / ![]() Theophylline levels Ciprofloxacin /
Theophylline levels Ciprofloxacin / ![]() Theophylline
plasma;
Theophylline
plasma; ![]() possibility of side effects Corticosteroids
/
possibility of side effects Corticosteroids
/ ![]() Theophylline levels Digitalis /
Theophylline levels Digitalis / ![]() Digitalis
toxicity Disulfiram /
Digitalis
toxicity Disulfiram / ![]() Theophylline levels Ephedrine and other
sympathomimetics /
Theophylline levels Ephedrine and other
sympathomimetics / ![]() Theophylline levels Erythromycin /
Theophylline levels Erythromycin / ![]() Theophylline
effect R/T
Theophylline
effect R/T ![]() liver metabolism Ethacrynic acid / Either
liver metabolism Ethacrynic acid / Either ![]() or
or ![]() theophylline
levels Furosemide / Either
theophylline
levels Furosemide / Either ![]() or
or ![]() theophylline levels Halothane /
theophylline levels Halothane / ![]() Risk
of cardiac arrhythmias Interferon /
Risk
of cardiac arrhythmias Interferon / ![]() Theophylline
levels Isoniazid / Either
Theophylline
levels Isoniazid / Either ![]() or
or ![]() theophylline levels Ketamine / Seizures of
the extensor-type Ketoconazole /
theophylline levels Ketamine / Seizures of
the extensor-type Ketoconazole / ![]() Theophylline levels Lithium /
Theophylline levels Lithium / ![]() Lithium
effect R/T
Lithium
effect R/T ![]() rate of excretion Loop diuretics /
rate of excretion Loop diuretics / ![]() Theophylline
levels Mexiletine /
Theophylline
levels Mexiletine / ![]() Theophylline levels Muscle relaxants,
nondepolarizing /
Theophylline levels Muscle relaxants,
nondepolarizing / ![]() Muscle relaxation Oral contraceptives /
Muscle relaxation Oral contraceptives / ![]() Theophylline
effect R/T
Theophylline
effect R/T ![]() liver metabolism Phenytoin /
liver metabolism Phenytoin / ![]() Theophylline
levels Propofol /
Theophylline
levels Propofol / ![]() Sedative effect of propofol Quinolones /
Sedative effect of propofol Quinolones / ![]() Theophylline
levels Reserpine /
Theophylline
levels Reserpine / ![]() Risk of tachycardia Rifampin /
Risk of tachycardia Rifampin / ![]() Theophylline
levels St. John’s wort / Possible
Theophylline
levels St. John’s wort / Possible ![]() theophylline plasma levels R/T
theophylline plasma levels R/T ![]() metabolism
Sulfinpyrazone /
metabolism
Sulfinpyrazone / ![]() Theophylline levels Sympathomimetics /
Theophylline levels Sympathomimetics / ![]() Theophylline
levels Tetracycline /
Theophylline
levels Tetracycline / ![]() Risk of theophylline toxicity Thiabendazole
/
Risk of theophylline toxicity Thiabendazole
/ ![]() Theophylline levels Thyroid hormones /
Theophylline levels Thyroid hormones / ![]() Theophylline
levels in hypothyroid clients Tobacco smoking /
Theophylline
levels in hypothyroid clients Tobacco smoking / ![]() Theophylline
effect R/T
Theophylline
effect R/T ![]() liver metabolism Troleandomycin /
liver metabolism Troleandomycin / ![]() Theophylline
effect R/T
Theophylline
effect R/T ![]() liver metabolism Verapamil /
liver metabolism Verapamil / ![]() Theophylline
effect Zafirlukast / Possible
Theophylline
effect Zafirlukast / Possible ![]() theophylline levels
theophylline levels
Additional Drug Interactions: Possible ![]() Serum
theophylline levels when used with zafirlukast.
Serum
theophylline levels when used with zafirlukast.
How Supplied: Capsule: 100 mg, 200 mg; Capsule, extended
release: 50 mg, 75 mg, 100 mg, 125 mg, 130 mg, 200 mg, 250 mg, 260 mg, 300
mg; Elixir: 80 mg/15 mL; Solution: 80 mg/15 mL; Syrup: 80
mg/15 mL, 150
mg/15 mL; Tablet: 100 mg, 125 mg, 200 mg, 250 mg, 300 mg; Tablet, extended release: 100 mg, 200 mg, 250 mg, 300 mg, 400 mg, 450 mg, 500 mg, 600 mg
Dosage
·
Capsules, Elixir, Oral
Solution, Syrup, Tablets Bronchodilator,
acute attacks, in clients not currently on theophylline therapy.
Adults and children over 1 year of age, loading dose: 5 mg/kg. Maintenance,
Adults, nonsmoking: 3 mg/kg q 8 hr; Older clients, those with cor
pulmonale: 2 mg/kg q 8 hr. Clients with CHF: 1-2 mg/kg q 12 hr; Children,
9-16 years of age and young adult smokers: 3 mg/kg q 6 hr; Children, 1-9
years of age: 4 mg/kg q 6 hr.
Infants, 6-52 weeks, initial maintenance dose: Calculate as follows:
[(0.2 x age in weeks) + 5] x kg = 24 hr dose in mg. For infants up to 26 weeks,
divide into q 8 hr dosing; for infants 26-52 weeks, divide into q 6 hr dosing.
Bronchodilator, acute attacks, in clients currently receiving theophylline.
Adults and children up to 16 years of age: If possible, a serum
theophylline level should be obtained first. Then, base loading dose on the
premise that each 0.5 mg theophylline/kg lean body weight will result in a
0.5-1.6-mcg/mL increase in serum theophylline levels. If immediate therapy is
needed and a serum level cannot be obtained, a single dose of the equivalent of
2.5 mg/kg of anhydrous theophylline in a rapidly absorbed form can be given.
Chronic therapy, based on anhydrous theophylline.
Adults and children, initial: 16 mg/kg/24 hr, up to a maximum of 400
mg/day in three to four divided doses at 6-8-hr intervals; then, dose
can be increased in 25% increments at 2-3 day intervals up to a maximum, as
follows: Adults and children over 16 years of age: 13 mg/kg, not to
exceed 900 mg/day; 12-16 years: 18 mg/kg/day; 9-12 years: 20
mg/kg/day; 1-9 years: 24 mg/kg/day.
·
Extended Release Capsules
and Tablets Bronchodilator, chronic
therapy, based on equivalent of anhydrous theophylline.
Adults, initial: 6-8 mg/kg up to a maximum of 400 mg/day in three to
four divided doses at 6-8-hr intervals; then, dose may be increased, if
needed and tolerated, by increments of 25% at 2-3 day intervals up to a maximum
of 13 mg/kg/day or 900 mg/day, whichever is less, without measuring serum
theophylline. Pediatric, over 12 years of age, initial: 4 mg/kg q 8-12
hr; then, dose may be increased by 2-3 mg/kg/day at 3-day intervals up
to the following maximum doses (without measuring serum levels): 16 years
and older: 13 mg/kg/day or 900 mg/day, whichever is less; 12-16 years: 18
mg/kg/day.
·
Elixir, Oral Solution,
Syrup Neonatal apnea.
Loading dose: Using the equivalent of anhydrous theophylline administered
by NGT, 5 mg/kg; maintenance: 2 mg/kg/day in two to three divided doses
given by NGT.
•IV Bronchodilator, acute attacks.
See above doses using PO products.
Steroids comprise five general groups of complex organic compounds which are produced in the adrenal cortex. The group that has clinical relevance to respiratory therapy is the glucocorticoids. Cortisol and glucocorticoids regulate the metabolism of carbohydrates, fats, and proteins to generally increase levels of glucose for energy to be used by the body. That is why cortisol and its analogues are called glucocorticoids.
One of the major therapeutic effects seen with analogues of natural adrenal cortical hormone hydrocortisone is an antiinflammatory action. Glucocorticoid analogs are used for their antiinflammatory effects in treating asthma, which is basically a disease in which there is chronic inflammation of the airway wall that causes airflow limitation and hyperresponsiveness to a variety of stimuli.
Steroids can be administered orally, intravenously (IV), or aerosolized for respiratory symptoms. The IV drug of choice is usually hydrocortisone or methylprednisolone. Oral drug of choice is prednisone or prednisolone. Aerosolized corticosteroid preparations that have antiinflammatory effectiveness in the treatment of asthma include hydrocortisone, cortisone, prednisone, prednisolone, and methylprednisolone.
In treating respiratory diseases, steroids are administered orally for more significant exacerbations of bronchospasms, and by IV for serious bronchospasm. However, the potential side effects of systemic administration of corticosteroid treatments are well recognized, and include:
· HPA suppression
· immunosuppression
· increased glucose levels
· fluid retention
· hypertension
· increased white blood cell count
· peptic ulcer
· osteoporosis
· psychiatric reactions
· growth retardation
· myopathy of skeletal muscle
· cataract formation
· dermatologic changes
The quantity, severity, and frequency with which these complicating side effects appear when systemic steroid treatments are used have provided the motivation for transferring patients to aerosolized, inhaled steroids whenever possible. The introduction of synthetic analogues of hydrocortisone, which have a high topical antiinflammatory activity, have paved the way for effectively using aerosolized steroids with little systemic side effects. These drugs include: beclomethasone, triamcinolone, flunisolide, budesonide, and fluticasone.
While the switch to inhaled aerosol steroids has reduced the number of side effects previously seen with systemic steroid therapy, there remain some local and system side effects that need to be considered by caregivers. The following table illustrates the potential hazards and side effects associated with using inhaled aerosol corticosteroids:
|
Systemic |
Local (topical) |
|
Adrenal insufficiency1 |
Oropharyngeal fungal infections |
|
Extrapulmonary allergy1 |
Dysphonia |
|
Acute asthma1 |
Cough, bronchoconstriction |
|
HPA suppression (minimal, dose dependent) |
Incorrect use of MDI |
|
Possible growth retardation |
Possible osteoporosis in asthmatic patients |
|
1 Following transfer from systemic corticosteroid therapy. |
|
Aerosol corticosteroid therapy is currently considered clinically indicated for:
· control of asthma
· treatment of related steroid-responsive bronchospastic states not controlled by other therapies
· control of seasonal allergic or non-allergic rhinitis
The increased emphasis on viewing asthma as primarily a disease of inflammation leading to bronchial hyperresponsiveness has shifted the indicated use of inhaled aerosol steroids from second or third line to front line, primary therapy. The NIH’s 1997 Guidelines for Diagnosis and Management of Asthma now identify aerosolized corticosteroids as long-term control therapy rather than as quick-relief for acute, severe asthmatic episodes.
The late-phase response of allergic induced bronchospasm can be mitigated or prevented by early application of inhaled steroids. In general, steroids do not replace bronchodilators, but should be used to supplement them.
Dexamethasone (Decadron) is one of the first successfully aerosolized agents (available since 1976) for inhalation, and it has an antiinflammatory potency of 30 times that of hydrocortisone. However, because it does not potentiate the beta2 receptors and the systemic side effects associated with it, the use of aerosolized dexamethasone has declined in favor of newer medications. It is available as a nasal spray (Turbinaire) and MDI (Respihaler). Each activation of the MDI delivers approximately 0.1 mg. Adult dose is 3 puffs TID or QID, up to a maximum of 12 per day. Pediatric dose is 2 puffs TID or QID, up to 8 per day. Each MDI delivers about 170 puffs.
Beclomethasone dipropionate (Vanceril, Beclovent) was the second aerosolized corticosteroid made available in this country, and is indicated for controlling intrinsic, extrinsic, and mixed asthma in patients over six years of age who require steroid therapy. The drug’s success as an aerosol in reducing or replacing the use of systemic steroids is due to its high topical to systemic activity ratio (approximately 500 times that of dexamethasone). Beclomethasone has also been reported to minimize symptoms of perennial rhinitis in patients susceptible to antigens such as pollen.
An aerosol dose of 400 mcg of beclomethasone is approximately equivalent to 5-10 mg of oral prednisone. Adult dose is 0.5 to 1 mg QID. For the Vanceril MDI, one to four puffs are given 3-4 times a day. Each puff delivers about 42 mcg. The maximum daily adult dose is 840 mcg, with the pediatric dosage being about half of this. Asthmatic symptoms decrease in about 80% of patients concurrent with an improvement in pulmonary function. This occurs without the systemic side effects of oral steroids, although Candidiasis has been reported in some cases.
Betamethasone is a synthetic corticosteroid indicated for severe inflammation, immunosuppression, or adrenocortical insufficiency. Its duration of action is similar to dexamethasone, and has about 75% of the potency of beclomethasone. Daily dosage is 4 applications of 200 mcg each.
Triamcinolone Acetonide (Azmacort) an aerosol that is also topically active, and was available as Kenalog and Aristocort prior to its release as an aerosol. Available in an MDI preparation with a built-in spacer device, inhalations doses of about 100mcg, four times daily allow most steroid-dependent asthmatics to stop taking oral steroids. Aerosolized triamcinolone can cause hoarseness, voice weakness, and oropharyngeal candidiasis; however, rinsing the mouth and gargling after use generally prevents these side effects.
Flunisolide (AeroBid), another topically active MDI-packaged aerosol, is similar to triamincolone in potency, but is longer acting. Like beclomethasone, it shows a peak plasma level after inhalation between 2 and 60 minutes, indicating good absorption from the lungs. Because it is more potent than many steroids, its recommended dosage is reduced: two inhalations (250 mcg each) twice daily for adults, with half of this recommended for pediatric patients.
Fluticasone propionate (Flovent, Flonase) is a further analogue of previous agents with high topical potency, synthesized in order to avoid systemic side effects. It is part of androstane analogues which has a very weak HPA inhibitory activity, but high antiinflammatory effect. Available as a nasal spray and in MDI form in three different strengths, recommended adult dosage is 44-220 mcg BID. Fluticasone propionate is contraindicated in patients with acute status asthmaticus, respiratory tract infections, or tuberculosis.
Budesonide is a topically active inhaled corticosteroid less potent than fluticasone, but greater than beclomethasone. After inhalation with a spacer device, peak plasma concentrations occur between 15-45 minutes with a half-life of 2 hours, and there appears to be minimal metabolism in the lung (about 70% of inhaled dose reaches the circulation). The recommended adult dosage is one puff (200 mcg) BID. Half this dose is used for children using a 50 mcg MDI. Budesonide may be given up to 3 puffs (600 mcg) BID, and is available as a nasal aerosol for treating allergic rhinitis.
Hydrocortisone (variety of trade names including Hydrocortone, Acticort, and Cetacort) is a steroid that can be administered orally, parenterally, and only rarely by aerosol. Its plasma concentrations of 100‑150 mcg/ml are generally high enough to diminish the symptoms of status asthmaticus. The adult daily dose can range from 300 to 2000 mg.
Prednisone (Deltasone) is an oral steroid in tablet form that has an anti‑inflammatory potency 3-4 times that of hydrocortisone. Its onset of action is somewhat delayed because it becomes active only after its been converted to prednisolone in the liver. As an aerosol, it is completely ineffective. Indications include severe inflammation or immunosuppression, nephrosis, or acute exacerbations of multiple sclerosis. Adult dosage is PO 1.5-2.5 BID-QID, followed by once daily or QOD, with maintenance dosage up to 250 mg daily.
Prednisolone (numerous trade names include Prelone, Predicort, Key-Pred) is an intermediate acting synthetic steroid that is available by injection, orally, and is rarely aerosolized. Anti‑inflammatory potency is 3‑4 times that of hydrocortisone but it takes longer to reach its peak effect. The half‑life is 2 to 4 hours and pharmacological effects last up to 36 hours. Usual adult dose is PO 2.5-15 mg BID-QID; IM 2-30 mg Q 12 hours; IV 1-30 mg daily..
Methylprednisolone (Duralone, Medralone, Depopred, et al) has 4-5 times the anti‑inflammatory potency of prednisolone, and is used frequently because it has little effect on electrolyte balance. Available orally, but is usually administered intravenously. Methylprednisolone is indicated for severe shock, status asthmaticus, ARDS, and aspiration pneumonia. Onset of action is rapid, half‑life is 78‑188 minutes, and pharmacological effects remain for up to 36 hours. Dosage varies depending upon symptoms.
Asthma is essentially an inflammatory disorder of the airways, in which allergic stimuli often trigger IgE-mediated mast cell release of mediators of inflammation. Airway reactivity can be triggered by such nonspecific stimuli as cold air or dust. Allergic inflammation of the airway is a product of an immune response, and the T-lymphocyte plays a central role in attracting mast cells and eosinophils, which in turn release mediators that attract other cells and damage epithelial cells.
The clinical result of asthma is a chronic persistent inflammation of the airway, coupled with occasional acute episodes of wheezing and airway obstruction caused by bronchoconstriction, mucosal swelling and mucus secretion. There are drugs available to inhibit the mediators of inflammation, including cromolyn sodium, nedocromil sodium, zafirlukast, and zileuton. These agents, sometimes referred to as mediator modifiers, are prophylactic and are intended to assist the management of chronic asthma, not to relieve acute airway obstruction or provide bronchodilation in an acute asthma attack. Patients who show an improvement in bronchospastic symptoms with steroids may benefit from mediator modifier
Cromolyn sodium (Disodium Cromoglycate) is considered an antiasthmatic, antiallergic, and mast cell stabilizer. Cromolyn is available as a dry powder inhaler, a nebulizer solution, and an MDI. It does not block cholinergic, muscarinic receptors, and has no intrinsic bronchodilating capability. Pretreatment with inhaled cromolyn sodium results in inhibition of mast cell degranulation, thereby blocking release of the chemical mediators of inflammation. While the dosage varies at the discretion of the physician, the usual adult dose of cromolyn is 20 mg TID or QID.
Nedocromil sodium (Tilade) is another prophylactic drug used in the management of mild to moderate asthma. It exerts its antiinflammatory and antiasthmatic effect by inhibiting the activation and activity of multiple inflammatory cells, including mast cells, eosinophils, airway epithelial cells, and sensory neurons. Available as an MDI with 1.75 mg per actuation, the recommended dosage for maintenance therapy in asthma is two inhalations 4 times a day.
Zafirlukast (Accolate) is a relatively new (approved for use in U.S. in 1996) prophylactic agent that acts on a portion of the inflammatory process as a leukotriene receptor antagonist, preventing the inflammatory response of airway contractility, vascular permeability, and mucus secretion caused by certain leukotrienes.
While it has been in use a relatively short period of time, Zafirlukast gives evidence of being effective in preventing bronchoconstriction and other asthmatic airway responses, against LTD4-induced constriction, allergen, exercise, and cold air challenge. Side effects have included headache, respiratory infection, nausea, diarrhea, generalized and abdominal pain.
Zileuton (Zyflo) also new (approved in 1997), inhibits the 5-lipoxygenase enzyme which would otherwise catalyze the formation of leukotrienes from arachidonic acid. It is indicated for the prophylaxis and chronic treatment of asthma in patients over 12 years of age, and has been effective in attenuating the asthma response to allergen challenge, cold air, and aspirin challenges. It is available in a 600 mg tablet form, with dosage being recommended at one tablet 4 times daily.
There are at least four classes of drugs which provide antiinflammatory activity within the arachidonic acid cascade, and which are being investigated for possible use in assisting the treatment of asthma (some only have code names at this time):
1. Cysteinyl LT antagonists
Zafirlukast, ICI-204, 219 (Accolate)
Pobilukast, SKF 104353-Q
Pranlukast ONO-1078
Verlukast MK-679
2. 5-lipoxygenase inhibitors
Zileuton, A-64077 (Zyflo)
Z-02128
3. FLAP binding inhibitors
M-886
MK-0591
BAY x1005
4. LTB4 antagonists
ONO-4057
U-75,302
Inhibitors of 5-lipoxygenase either inhibit the enzyme directly or bind to a membrane protein called FLAP. FLAP then combines with 5-lipoxygenase to inhibit leukotriene synthesis. Zileuton is an agent that directly inhibits 5-lipoxygenase. While there are still questions regarding the usefulness of blocking the synthesis or activity of a single family of mediators such as leukotrienes, there has been a high efficacy in clinical trials of these drugs that supports the thesis that these may prove useful in the future.
Prostaglandins are synthesized in all tissues, and the three that are of substantial interest in respiratory therapy are PGE1, PGE2, AND PGF2A. The first two because they cause relaxation of bronchial smooth muscle, and the latter because it causes contraction of bronchial muscle. Prostaglandins, unlike adrenergic or anticholinergic drugs, act directly on smooth muscle.
Prostaglandins are used for vasodilation of the pulmonary vascular bed in patent ductus arteriosus (PDA). Increased pulmonary vascular resistance in PDA helps maintain a shunt through the patent ductus, and lowered resistance allows more blood to flow through the pulmonary system and less through the ductus. It is important to release the prostaglandin via a line located distal to the ductus, otherwise the ductus becomes dilated and the problem is worsened.
Anticholinergics or parasympatholytic bronchodilators, which are also often referred to as antimuscarinics because they act at the muscarinic receptors of the parasympathetic nervous system, achieve bronchodilation through a different pathway in the autonomic nervous system. As a result, anticholinergics can be used either alone or in combination with beta adrenergics.
Because they tend to decrease secretion production, drying of the airways can be a problem if significant doses are administered. Additional side-effects can include: drying of the mouth and skin, blurred vision, and an increase in speech, swallowing, and micturition problems. Among these drugs, the most common include:
Atropine sulfate which has traditionally been the model antimuscarinic bronchodilator agent used in the treatment of airway disease. It has an additive effect to the Beta adrenergic agonists when given together. However, the development and increased use of adrenergic drugs has tended to gradually displace atropine as a bronchodilator.
Atropine is available as a nebulized solution administered via injection or aerosol (Dey-Dose). Because it is a tertiary ammonium compound, atropine is readily absorbed by aerosol, and side effects are seen in the dosages required for effective bronchodilation. Duration and incidence of side effects are therefore dose dependent. Normal inhaled dose for atropine is around 0.025 mg/kg for adults (2.5 mg per 24 hours maximum), with onset in 15 minutes, peak at .5-1.0 hour, and duration 3-4 hours. Atropine is also available in tablets and elixirs.
Ipratropium Bromide (Atrovent) is approved specifically for the maintenance treatment of airflow obstruction in COPD. It is considered a first-line medication for COPD patients, particularly those with chronic bronchitis. It is currently available in two formulations for bronchodilator use: an MDI with 18 mcg per puff, and a nebulizer solution of 0.02% concentration in a 2.5 ml vial, providing a 500 mcg dose per treatment. Usual adult dose is 2 puffs QID via MDI (12 puffs per 24 hours maximum).
The side-effects of antimuscarinics are minimal or absent in most patients using ipratropium, and systemic absorption via the GI tract and mucosal surface is also minimal. It has an additive effect to the Beta adrenergic agonists and, provides better bronchodilation for many COPD patients. It should be delivered prior to Beta agonists in order to achieve the best results.
Some other antimuscarinics include:
· Glycopyrrolate, a derivative of atropine which is usually administered parenterally, is used as an alternative to atropine because it has fewer ocular or central nervous system side effects. The injectable solution has been nebulized into a 1 mg dose for bronchodilation.
· Oxytropium bromide is a derivative of scopolamine that has been investigated as an aerosolized anticholinergic bronchodilator in patients with obstructive airway disease. An MDI-delivered dose of 200 mcg provides a peak effect on FEV1 within 1-2 hours, with a duration of 6-8 hours. Normal dosage is 2 puffs BID or TID, and systemic anticholinergic effects are rare. Side effects include local irritation of the throat and nose, dry mouth, nausea, wheeze, cough, and a tightness in the chest in a few patients.
· Tiotropium bromide is still an investigational, long-acting antimuscarinic drug that may offer an attractive and safe alternative for maintenance treatment of COPE, and protection for nocturnal asthma.
One of the major clearing and defense mechanisms of the airway- conducting zone of the lung is referred to as the mucociliary system. Mucokinetics is primarily concerned with the movement of mucus in the respiratory tract, and the overall effectiveness of the mucociliary system.
The effectiveness of the system depends largely on the interactions between the cilia and the mucus blanket, whose composition represents a delicate balance between the secretions of the goblet cells and bronchial glands. Failure of this system results in mechanical obstruction of the airway with thickened, adhesive secretions. A significant slowing of mucus transport is associated with the abnormal mucociliary function seen in bronchitis, asthma, and cystic fibrosis. Mucokinetic therapy is designed to maintain or improve functioning of the mucociliary mechanism, thereby promoting clearance of respiratory tract secretions and reducing the potential for infection.
Mucokinetic/mucolytic agents achieve their effect through a variety of ways, including:
· acting directly upon the chemical constituents of mucus to decrease mucus viscosity or tenacity
· diluting the mucus resulting in disadherence from the airway
· making the ciliary action more effective by replenishing or increasing the watery sol layer of mucus
· directly stimulating the cilia
· stimulating the bronchial glands to produce secretions that are less viscous
· a combination of several of these actions
Water is one of the most important and safest agents used to modify the character of respiratory tract secretions. Consumption of adequate amounts of water is crucial for optimal functioning of the respiratory system, and is even more important for a patient who has difficulty mobilizing bronchial secretions. Water can also be vaporized or aerosolized for delivery to patients whose upper airway has been bypassed by intubation. However, caution should be taken to avoid either over- or under-hydration if normal mucus is to be achieved. This is especially true for patients on fluid restriction or with congestive heart failure.
Saline (NaCl) is commonly nebulized for diluting the mucus and enhancing clearance, and small amounts (1-3 cc) of normal saline (0.9% NaCl) are used to dilute other medications for aerosolization. Like water, saline is absorbed into the sol layer to disadhere mucus from the airway. Many clinicians prefer to use half-normal saline (0.45% NaCl) for mucosal hydration, especially with ultrasonic nebulization, because the evaporation of water from droplets of this solution results in a solute concentration like that of normal saline.
Hypertonic saline solutions (1-15% NaCl) are the agents of choice for sputum inductions, because its elevated osmolarity can result in increased movement of fluid into the bronchorrhea. These solutions are obviously contraindicated for sodium-restricted patients.
Propylene Glycol, which is both a solvent and hygroscopic agent, is used to stabilize aerosol droplets from bronchodilators and to inhibit the potential for bacterial growth. It is safe in low concentrations, creating a soothing effect on the respiratory mucosa. In concentrations greater than 5%, it is often used to induce sputum.
Ethyl Alcohol (ETOH) is a wetting agent that has been used to destabilize the alveolar plasma exudates occurring in cardiogenic pulmonary edema. It acts to destabilize the froth observed in the alveoli and bronchioles in cardiogenic pulmonary edema. Normally, 5-15 mL of 30-50% ETOH is vaporized by positive pressure. Temporary irritation of the airway mucosa is the only side effect experienced in this treatment.
True mucolytics are drugs intended to control mucus and bronchial secretions. The two primary agents approved for administration as aerosols to treat abnormal pulmonary secretions are acetylcysteine and dornase alfa. Both act to disrupt the disulfide bond in mucus and break down DNA materials in airway secretions.
Acetylcysteine (Mucomyst) is an aerosolized medication indicated for treatment of the thick, purulent, viscous mucus secretions that can occur in COPD, especially chronic bronchitis, tuberculosis, cystic fibrosis, and acute tracheobronchitis. It is also administered orally as an antidote to reduce hepatic injury with overdoses of acetaminophen, and is designated as an orphan drug.
Aerosol doses of Mucomyst are available in either 10 or 20% solutions, and normal dosage with 20% solution is 3-5 ml TID or QID, and 6-10 ml TID or QID with the 10% solution. The most serious potential side effect is bronchospasm, especially with hyperreactive airways seen in asthmatics, so using bronchodilators mixed with acetylcysteine or administer previously by MDI or nebulizer is recommended. Other potential side effects include stomatitis, nausea, and rhinorrhea.
Dornase Alfa (Pulmozyme) is an orphan drug that was produced by recombinant DNA techniques, and is indicated for the treatment and management of the viscid respiratory secretions seen in patients with cystic fibrosis (CF). In CF patients, Pulmozyme helps reduce the frequency of respiratory infections requiring parenteral antibiotics, and generally improves their overall pulmonary function.
Pulmozyme available as a single use ampule, with 2.5 mg drug in 2.5 ml of clear solution. Recommended dosage is 2.5 mg daily, delivered by nebulizers specifically approved for this use. Few side effects have been observed, including voice alteration, pharyngitis, laryngitis, rash, chest pain, and conjunctivitis.
Sodium bicarbonate (NaHCO3) helps break up large mucoid molecular chains because of its alkalinity. Some patients benefit from occasional aerosolized 2% sodium bicarbonate, which is a readily available solution for home use by simply putting a teaspoonful of the soda in a cup of sterile water. However, with the availability of more potent mucolytics like acetylcysteine, it is rarely used.
In addition to these most commonly used mucus-controlling agents, other mucoactive agents that have been or are now being explored include:
Beta andrenergic agonists can aid in mucokinesis, possibly by increasing the beat frequency of cilia. Active transport of the chloride ion into the airway lumen, augmented with a resulting water flux, may produce a less viscous, thinner mucus and enhance ciliary movement.
S-Carboxymethylcysteine (Mucodyne), an oral mucokinetic investigated in Britain, decreases sputum viscosity in vitro, but is not considered effective for mucolysis when administered orally. It is chemically related to garlic, a common home remedy mucokinetic, and other home remedies for mucokinesis including chicken soup, horseradish, pepper, and mustard.
Glyceryl guaiacolate, which is generally considered an expectorant, also has shown potential for improving mucociliary clearance in chronic bronchitis.
Potassium iodide, which is also generally considered an expectorant, has also shown potential for decreasing mucus elasticity, but has also shown a potential for harmful effects on cilia.
Sodium 2-mercaptoethane sulfonate, a compound containing a sulfhydryl group that is being explored in Britain, acts similar to acetylcysteine in reducing mucous viscosity.
Several enzymes have been shown to reduce the viscosity of mucus by breaking down the mucoprotein and deoxyribonucleic acid, which contribute to mucus viscosity. One example is the previously discussed dornase alfa. These continue to be explored as mucus controlling agents, but to date have generally proven to be too costly, irritating, and toxic.
Ambroxol is another orally administered drug that has been investigated as a mucokinetic agent. It stimulates ciliary beat frequency, but its ability to increase mucociliary clearance by result from stimulation of pulmonary surfactant or bronchial secretion.
Given the nature of inflammation in disease states of mucus hypersecretion, the use of mucolytic agents alone is not considered an adequate program of mucus control. Other therapeutic options that are considered necessary for controlling mucus hypersecretion include:
· Remove causative factors where possible, including cessation of smoking, and avoidance of pollution and allergens.
· Optimize tracheobronchial clearance by using a bronchodilator, taking bronchial hygiene measures (like hydration, coughing, deep breathing, postural drainage), using mucolytics and expectorants.
· Reduce inflammation by treating infection with antibiotics, and using corticosteroids.
The number of medications available for dealing with the common cold and the coughs that accompany it is mind-boggling. Between the host of over-the-counter medications and those requiring a prescription, there is enough information to warrant a separate CEU. In this unit we will only discuss the most significant cough suppressants and expectorants. The medications that work centrally are designed to increase the cough threshold in the medullary cough center. Peripherally active medications inhibit the cough at the mucosa, usually by coating supraglottic receptors with a thick syrup. Expectorants are agents that facilitate removal of mucus from the lower respiratory tract, and their mechanisms include
· vagal gastric reflex stimulation
· absorption into respiratory glands to directly increase mucus production
· topical stimulation with inhaled volatile agents
Super Saturated Potassium Iodide (SSKI) is available under a variety of trade names, and has been used for quite a long time as an expectorant in asthma and chronic bronchitis. In sufficient orally administrated concentrations, it has a direct mucolytic effect, and an indirect effect on mucus viscosity by stimulating submucosal glands to produce more serous secretions. Iodide also stimulates the
gastropulmonary reflex, can stimulate ciliary activity, and has a mucolytic effect. In some individuals, iodides are associated with hypersensitivity reactions.
Five to ten drops (3-600 mg) in a glass of water given 34 times a day may be given, with the pediatric dosage being about half that amount. Patients can develop acne or rashes and long term use may disrupt thyroid function. SSKI is contraindicated for patients with thyroid disease.
Guaifenesin (Glycerol Guaiacolate), available in a variety of trade name products, is thought to reduce the adhesiveness and surface tension of mucus secretions when taken orally. Dosage for children 6-12 years old is 2-400 mg every 4 hours with a maximum of 2,400 mg in 24 hours. Dosage for children 2-6 years old, half of this may be given, and for even younger children, half again (50-100 mg every 4 hours).
Iodinated Glycerol (Organidin) is available as a tablet, solution, or elixir. The adult dose of the 5% solution is 20 drops in a liquid QID. The 30 mg tablet dose is 2 tablets QID. The 1.2% elixir dose is 1 teaspoon QID. Pediatric dose is one half the adult dose.
Chicken soup, flavored with garlic and curry has been suggested as a tasty and effective stimulant via the gastric reflex. Garlic’s major constituent is alliin, which has a structure similar to mucolytic drug S-Carboxymethylcysteine. Other spices that could have similar potential include Tabasco sauce, horseradish, and mustard.
Codeine sulfate is a popular ingredient in variety of brand name cough suppressants. Compared to morphine, it is less addictive, has much less respiratory depressant activity, and is much less likely to cause bronchospasm or constipation. In doses below 15 mg, codeine does not produce analgesia in adults, with doses in the 10-20 range there is an antitussive action, and doses above 30 mg, codeine produces analgesia. Dosage is 10-20 mg every 4-6 hours, not to exceed 120 mg in 24 hours. For children 6-12 years old, dosage is 2.5-5 mg every 4-6 hours.
Benzonatate (Tessalon) is a nonnarcotic that has a local anesthetic effect with topical application. It acts on the sensory vagal receptors in the upper airway, and is though to inhibit the transmission of the afferent (cough and gag) impulse to the motor nerves through the medulla.
Dextromethorphan hydrobromide is a popular nonnarcotic antitussive that is available in a variety of brand name products, and is popular because it has no analgesic, respiratory depression, or addictive properties. Cough suppression is comparable to codeine. Dosage is 10-30 mg Q 4-8 hours up to a maximum of 120 mg in 24 hours. For children 6-12 years old, dosage is 5-10 mg Q 4 hours. For children 2-5 years old, dosage is 2.5-7.5 mg Q 4-8 hours.
Diphenhydramine hydrochloride, available in a host of brand name products (including Benadryl, Sominex, and Maximum Strength Nytol), is an antihistamine with antitussive properties, and is available in tablets, capsules elixirs, injections, lotions, and syrups. It can cause sedation and has anticholinergic effects. Adult dosage PO is 25-50 mg Q 4 hours, not to exceed 400 mg/day, and pediatric dosage is one-half that.
Hydrocodone (Hycodan Syrup) produces an antitussive effect with a dose of approximately 5 mg. However, it is an addictive derivative of opium, more potent than codeine, and can cause respiratory depression.
While there is still no cure for the common cold, that hasn’t stopped pharmaceutical companies from producing a bewildering number of compounds that purport to treat and even cure colds. The ingredients in these cold remedies include adrenergics, antihistamines, expectorants, and antitussives. Since most of these remedies are available over-the-counter, there is a very real potential for overdosing. Patients need to be cautioned that just because no prescription is needed, that doesn’t mean the compounds are not both powerful and potentially hazardous to their health.
Respiratory infections caused by bacterial, fungal, protozoal, and viral organisms occur in patients with pneumonia, acute and chronic bronchitis, bronchiectasis, sinusitis, and cystic fibrosis. Antibiotics represent one of the most commonly used anti-infective agents in the respiratory therapist’s arsenal.
The term antibiotic means a substance that is produced by microorganisms (bacteria, fungi, molds) that is capable of inhibiting or killing bacteria and other microorganisms. The mechanisms by which antibiotics inhibit or kill microorganisms include:
· Inhibition of cell wall synthesis. Bacterial cells have rigid protective walls, without which they explode. Antibiotics that inhibit bacterial cell wall synthesis include penicillins, bacitracin, cephalosporins, vancomycin, and cycloserine.
· Alteration of cell membrane permeability. Disruption of the cell’s membrane function upsets the necessary flow and storage of cell material required for growth. Membranes of certain bacteria and fungi are especially susceptible to antibiotics such as polymyxins.
· Inhibition of protein synthesis. Antibiotics that interfere with the ribosome’s ability to synthesize needed proteins include: chloramphenicol, tetracycline, erythromycin, lincomycin, streptomycin, kanamycin, and gentamicin.
· Inhibition of nucleic acid synthesis. Antibiotics that attach to the DNA strands and block further DNA replication or formation of messenger RNA include: fluoroquinolones (like ciprofloxacin), trimethoprim, sulfonamides, and rifampicin.
Antibiotics are usually administered systemically, but several (carbenicillin, gentamicin, streptomycin) have been aerosolized for localized infections (lung abscess and bronchiectasis). Tobramycin has been used for chronic infections in cystic fibrosis. Parenteral medications are often ineffective in lung infections because the presence of edema, fibrosis, or thick exudates limit diffusion of the drug into the lung.
Aerosolized antibiotics also may be useful when infections appear resistant to systemic therapy. However, aerosolized antibiotics should be considered a supplement to systemic therapy, not a replacement. They are probably most useful for stubborn gram-negative infections.
Results on aerosolized antibiotics have been mixed, and there are several disadvantages to aerosolized antibiotics, including:
· Bronchospasm is very common.
· Some antibiotics are inactivated by DNA and enzymes found in mucus.
· Doses for aerosolized administration have not been clearly established.
· Resistant microorganisms are created, as mentioned above.
· Expensive equipment may be needed and considerable staff time required for administration.
Despite these above, aerosolized antibiotics may be considered for children with cystic fibrosis, fungal infections (pulmonary coccidioidomycosis, endobronchial histoplasmosis), and when systemic therapy appears ineffective or toxicity has been reached.
Penicillins, one of the oldest classes (dating back to the early 1940s), are considered the most important of the beta-lactam antibiotics. These bactericidal antibiotics are used for pneumococcal pneumonias, nonhospital-acquired aspiration pneumonia, and lung abscesses. The penicillin group is divided into three subgroups: the natural penicillins like penicillin G, penicillinase-resistant agents like methicillin, and the broad-spectrum penicillins like ampicillin.
Penicillin G is the agent of choice for streptococcus pneumoniae, streptococcus pyogenes, and nonpenicillinase-producing staphylococcus aureus. Intramuscular or intravenous delivery is the preferred route of administration for acute and serious pulmonary infections.
Bacterial resistance to penicillin is caused by the production of the enzyme penicillinase, so a subgroup of the penicillins was created that are resistant to penicillinase, including methicillin, nafcillin, oxacillin, and cloxacillin, which are effective against penicillinase-producing S-aureus.
Semisynthetic broad-spectrum penicillin derivatives used to treat gram-negative microorganisms include ampicillin, amoxicillin, carbenicillin, piperacillin, and ticarcillin.
Ampicillin, available in several trade names (including Omnipen, Principen, Ampicin) is indicated treating systemic infections, acute and chronic urinary tract infections, and meningitis. It is used for pneumococcal pneumonia, bronchitis, bacterial exacerbations of COPD, streptococcus pneumoniae, and haemophilus influenzae.
Amoxicillin, an extended-spectrum penicillin, is indicated for lower respiratory infections. It is used for the same and is closely related to ampicillin. Both may be given orally but amoxicillin achieves an effective plasma concentration lasting twice as long as ampicillin. Recommended dosage for adults and children weighing more than 20 kg is PO 250-500 mg Q 8 hours.
Carbenicillin is effective against pseudomonas and other gram-negative bacteria, but not as effective as ampicillin against gram-positive organisms. It is generally used with another antibiotic to prevent development of resistant strains, and dosage may be administered in aerosol form in a 1-3 gram dose. Side effects frequently seen with the penicillins consist mainly of hypersensitivity reactions, and rashes, fever, and anaphylactic shock can occur. Cerebral irritation and gastrointestinal upset can be seen with higher doses.
Cephalosporins are a group of antibiotics originally derived from a fungus in the late 1940s. Like penicillin, they act by inhibition of bacterial cell synthesis, but they are resistant to penicillinase. Cephalosporins are active against both gram-negative and gram-positive bacteria, and are effective against staphylococcal, streptococcal, and klebsiella pneumonias, along with proteus mirabilis and Escherichia coli. Their primary use in pulmonary disease is concurrent with gentamicin for undiagnosed sepsis and for cephalosporin-sensitive gram negative organisms.
Carbapenems are members of the beta-lactam group of antibiotics that act by inhibition of bacterial cell wall syntheses, and have a wide spectrum of activity against both gram-positive and gram-negative organisms.
Monobactam (Azactam) is a synthetic bactericidal that is effective against a wide range of gram-negative aerobic organisms.
Aminoglycosides, a group of agents derived from different species of Streptomyces, act by preventing and distorting bacterial protein synthesis. Gentamicin, amikacin kanamycin, tobramycin, and streptomycin are used for treating gram-negative bacillary pneumonias. Aminoglycosides are inhaled to control Pseudomonas infection in cystic fibrosis. Their most significant side-effect is damage to renal tubules.
Tetracyclines are derived from Streptomyces species, and can be bacteriostatic or bactericidal, depending on dosage. They interfere with protein synthesis, and are effective against streptococcus pneumoniae, haemophilus influenzae, and mycoplasma pneumoniae. Tetracyclines are used as a prophylactic and for acute exacerbations of chronic bronchitis. Milk and milk products interfere with their absorption from the gastrointestinal tract. Side-effects from using tetracycline include gastrointestinal irritation, vomiting and diarrhea, bone marrow depression and skin rashes. Since they are temporarily incorporated in the liver and kidneys, they should be used with caution, if at all, for patients with liver or renal disease.
Fluoroquinolones are synthetic quinolone derivatives with broad spectrum activity against bacterial activity. Examples include ciprofloxacin, norfloxacin, ofloxacin, enoxacin, and lomefloxacin. When orally administered, these drugs provide high lung bioavailability.
Sulfonamides are not classed as antibiotics, but were the first effective group of chemotherapeutic agents used to treat systemic bacterial infections. With the availability of many natural and semisynthetic antibacterial agents, their use has declined. They are still used to treat intestinal and urinary tract infections, but are generally longer used for treating gonococcal, staphylococcal, or streptococcal infections.
Trimethoprim-Sulfamethoxazole (TMP-SMX) is not an antibiotic, but a chemical agent produced in the laboratory. It is an antibacterial agent commonly used to treat respiratory infections, and is the drug of choice for treatment of P. carinii in AIDS patients. Side effects include rash, fever and leukopenia.
Polymyxins, B and E, are polypeptide antibiotics. Polymyxin B is effective against gram-negative organisms, particularly pseudomonas. It disturbs osmotic properties of the cell membrane in its action. It can be aerosolized for both pseudomonas and gram-negative bacteria colonizing the airway. Polymyxin B is usually given intramuscularly. Renal damage is the major problem with its use. When aerosolized, severe bronchospasm may result. It also can cause a neuromuscular blockade leading to respiratory paralysis. Polymyxin E is known as colistin and is similar to polymyxin B.
Erythromycins are macrolide antibiotics that are used for respiratory, genital, gastrointestinal tract, and skin/soft tissue infections. These agents bind to a site on the 50S ribosomal subunit of organisms, thereby acting to inhibit protein synthesis. They are drugs of choice in treating against gram-positive organisms including pneumococcus, mycoplasma pneumoniae, chlamydia psittaci, beta hemolytic streptococcus and some haemophilus influenzae. Erythromycin also has been used for Legionnaires disease, and for patients who are allergic to penicillin.
Erythromycin, one of the safest antibiotics, is available by oral, IM, or IV administration. Possible side effects include nausea, vomiting, diarrhea, phlebitis and pain on injection. Erythromycin is primarily used for community-acquired pneumonia. Clarithromycin and azithromycin have been effective in treating disseminated Mycobacterium avium-intracellulare in AIDS patients. Clindamycin is effective against some staphylococcus aureus, diplococcus, bacteroides and other gram positive organisms. Specific pulmonary indications for its use are aspiration pneumonias, empyema. and lung abscess. Side effects include diarrhea and skin rashes.
The body contains a variety of potentially pathogenic fungi that can cause local inflammation or disease if:
· a person becomes immunocompromised
· the balance of resident bacterial flora is suppressed by broad-spectrum antibiotics
· or if an inhaled corticosteroid is used without a reservoir device.
Drugs that are antifungal include amphotericin B, nystatin, and some newer azole antifungal agents.
Amphotericin B, which is administered by IV, has been the drug of choice for serious fungal infections since the 1950s, but its toxic effects such as nephrotoxicity, fever, and hypotension have limited its use. Nystatin is applied topically, and is effective against yeast-like infections.
First line agents to treat against tuberculosis include isoniazid, rifampin, pyrazinamide, ethambutol, and streptomycin. These primarily inhibit the growth of the mycobacterium tuberculosis, but can be bactericidal with proper dosage. Usually two or more of the above agents are given simultaneously for 6-9 months. Isoniazid is the most effective, and is believed to act as an antimetabolite in the bacilli. This inhibits most enzyme systems in the organism. Side-effects are directly related to dosage, duration of treatment, and increasing patient age. Complications include: mouth dryness, visual problems, headache, insomnia, constipation, anemia, orthostatic hypotension, skin rashes, seizures, peripheral neuritis and hepatitis. Vitamin B6 (pyridoxine) can decrease the neurotoxic effects.
Resistant strains develop rapidly when any of the above are given by themselves, so multidrug combinations are used to fight TB. Second-line antituberculosis drugs consist of capreomycin, kanamycin, ethionamide, paraaminosalicylic acid, and cycloserine.
Pentamidine Isethionate is an antiprotozoal drug which is active against Pneumocystis carinii pneumonia (PCP). It can be given either parenterally or via aerosol, and aerosolized pentamidine reaches significantly higher concentrations in the lung than when given by IV. Inhaled pentamidine (NebuPent) is specifically approved for the prevention of PCP in HIV-infected patients who:
· have a history of one or more episodes of PCP
· have a peripheral CD4+ lymphocyte count of 200/mm3, or less
The aerosol form has also been used in the treatment of acute episodes of PCP. Dosage approved for prophylaxis of PCP in AIDS subjects is 300 mg, given by inhalation once every 4 weeks. If there is a response to pentamidine, respiratory function may improve within 24-48 hours. Improvement generally takes between 2-8 days for most patients. Improvement in the chest X-ray may take a week or longer, particularly with an AIDS patient.
While pentamidine administered by aerosol has fewer side effects than it does when given parenterally, it still has the potential for side effects, including: cough and bronchial irritation, shortness of breath, bronchospasm and wheezing, conjunctivitis, rash, renal insufficiency. Using a beta adrenergic bronchodilator before inhaling aerosolized pentamidine can reduce or prevent local airway reaction.
The greatest danger of inhaling aerosol pentamidine exists when the patient coughs or removes the mouthpiece from their mouth while nebulization continues. Proper instruction of the patient on controlling aerosol particles by coughing into a tissue minimizes the former.
The antiviral drug Ribavirin (Virazole) is active against respiratory syncytial virus (RSV), influenza virus, and herpes simplex virus. It has been approved as an inhaled aerosol for treating some infants and children who have, or are at increased risk for, severe lower respiratory tract infections caused by RSV. The American Academy of Pediatrics’ recommendations for using Ribavirin include:
· For use to treat patients hospitalized with RSV lower respiratory tract disease, at high risk for severe/complicated RSV infection caused by the following:
· complicated congenital heart disease
· bronchopulmonary dysplasia, cystic fibrosis, or other chronic lung conditions
· premature infants
· those with immunodeficiency
· recent transplant recipients
· those receiving chemotherapy for malignancy
· infants who are severely ill (PaO2 <65 torr, SaO2 <90%, increasing PaCO2)
· patients on mechanical ventilation for RSV infection
· hospitalized infants at increased risk of progressing from mild to complicated course because of young age or underlying condition
Ribavirin is supplied as a powder of 6 grams per 100 ml vial. Sterile H20 is injected into the vial to achieve a concentration of 20 mg/ml (final total volume for administration is 300 ml). The mixture is good for 24 hours and then must be discarded. It is administered through a small particle aerosol generator (SPAG). The aerosol is delivered into an 02 hood, tent, or ventilator circuit.
Treatment with Ribavirin is usually for 12-18 hours per day for 3-7 days, and the package insert instructions should be consulted for more complete information. Side effects commonly seen include pulmonary function deterioration, and skin irritation from excess drug precipitation.
Surfactant is a surface-active agent that lowers surface tension. One of the most common examples is detergent. The term exogenous describing this class of drugs refers to the fact that surfactant preparations are obtained from outside the patient’s own body (i.e., from other humans, from animals, or synthesized in the lab).
Lung immaturity, which causes a lack of pulmonary surfactant is the primary problem in RDS. It results in high surface tension in the liquid-lined, gas filled alveoli. The clinical use of exogenous surfactants has traditionally been to replace the missing pulmonary surfactant of the premature, or immature, lung in respiratory distress syndrome (RDS) of newborns. These agents are also being investigated in treating adult respiratory distress syndrome (ARDS).
The clinical indications for exogenous surfactants are for the following applications:
· prevention of RDS in low birth weight infants
· prevention of RDS in other infants with evidence of immature lungs
· retroactive, or rescue treatment of infants with RDS
Exogenous surfactants act to replace and replenish a deficient endogenous surfactant pool in neonatal respiratory distress syndrome. Exosurf, colfosceril palmitate, is a protein-free, synthetic surfactant preparation. An indication for its use is the presence or risk of RDS. Dosage is 5 ml/kg of the reconstituted suspension, given as two divided doses of 2.5 ml/kg by direct tracheal administration. Details of preparing the suspension should be reviewed in the manufacturer’s literature.
Beractant (Survanta) is a modified natural surfactant that has similar indications as Exosurf, and the recommended dose is 100 mg/kg of birth weight. The primary complications in exogenous surfactant therapy are caused by the dosing procedure, and include airway occlusion, high PaO2 levels, over-ventilation, apnea, and pulmonary hemorrhage.
At the present time, medications do not play a major role in treating ARDS. However, there are two basic strategies for the using drugs to treat ARDS: improve/correct lung function and prevent/correct lung and systemic inflammation. Drugs for the former consist of almitrine, nitric oxide, surfactant replacement, and prostaglandin E1. For the latter, drugs consist of antioxidants, anticytokines, antiendotoxins, corticosteroids, and monoclonal antibodies.
Almitrine improves PaO2 and decreases PaCO2 in COPD patients through decreasing V/Q mismatch. In ARDS, almitrine has been effective in redistributing blood flow from areas of shunt to normal areas. It gives a similar increase in PaO2 to 10 cm H2O PEEP without the side effects of positive Pressure.
Nitric Oxide (NO) is a potent pulmonary vasodilator when inhaled. It has relatively no effect on the systemic circulation because it is inactivated by hemoglobin. Inhaled NO selectively improves perfusion of ventilated areas, and improves oxygenation in ARDS. Nitric oxide significantly decreases pulmonary artery pressure, decreases shunting and improves PaO2.
However, NO is a toxic component of air pollution, and exposure to it can result in pulmonary edema and unacceptable levels of methemoglobin. Not all patients respond to NO and it is impossible to predict which patients will respond. Effective doses of inhaled NO are less than 20 parts per million (ppm), and at doses less than 20 ppm, NO is considered relatively free of toxicity.
Prostaglandin E1 decreases systemic and pulmonary vascular resistance, decreases blood pressure, increases stroke volume, increases cardiac output, and increases heart rate. It may be useful to decrease pulmonary hypertension and improve cardiac function, but doesn’t appear to enhance survival in ARDS.
Antioxidants also may be of use in ARDS. N‑acetylcysteine has improved lung function, 02 delivery, and cardiac output in one study. A larger study showed no improvement in oxygenation, but an improvement in compliance. There was no difference in survival with the use of N-acetylcysteine.
Cytokines, such as interleukins, play a major role in causing the systemic effects associated with the sepsis syndrome. Clinical trials of anticytokines have been conducted. Studies of interleukin-1 receptor antagonist showed a minor but statistically insignificant improvement in mortality.
Antiendotoxins may prove useful, particularly in gram negative sepsis. Endotoxins produced by gram negative bacteria cause severe disturbances. Several antibodies against endotoxins have been studied. HA-lA showed an improvement in mortality in patients with gram-negative bacteremia.
Corticosteroids were tried in ARDS treatments in the past, but were relatively unsuccessful. Current emphasis is on the damaging potential of WBCs, however, if WBCs are interfered with by steroids, patients run the risk of increased infection. Steroids may be useful in minimizing fibrosis formation in the latter stages of ARDS. They are recommended if the patient is hypotensive from adrenal insufficiency. They also may be useful in aspiration, fat embolism, and chemical injuries to the airway.
Monoclonal antibodies are being studied to inhibit WBC-adhesion molecules, particularly those of the neutrophil. Neutrophil adhesion to tissue is a critical step in ARDS lung injury.
Use of ibuprofen has showed a decrease in the incidence and development of ARDS, increased rate of reversal, and improved survival. Some soluble protective agents against lung damage are tocopherol, ascorbate, and beta-carotene. These help protect membranes and other cellular elements from oxidant injury, however their value in ARDS remains unconfirmed.
Approximately 10% cigarette smokers per year try to quit. By the end of one year only about 20% of those “would-be-quitters” have been successful. The problem is that cigarettes create both a psychological and physiological dependence, and both need to be treated with behavioral and pharmacological therapies for maximum success. For the latter, there are nicotine and nonnicotine replacements. They are most effective when combined with appropriate behavior modification therapy.
Nicotine replacements come in the form of gum, patches, pills, nasal spray, and inhalers. Nicotine polacrilex gum has been used the longest. Nicotine patches are available in 16 and 24-hour forms. The 16-hour patch was created to prevent excess nicotine release during sleep. The patches are available in 21 mg, 14 mg, and 7 mg doses for weaning. Nicotine nasal spray and inhalers are uncommon at this time (1997). The pills are fairly new, and there is little data on success rates.
Nonnicotine replacements consist of CLONIDINE and BUSPIRONE. The primary use for clonidine is for hypertension, but it also relieves withdrawal symptoms from nicotine and the opiates. It is available as tablets or patches in doses of 0.1 to 0.3 mg/day. Buspirone is an antianxiety drug from the benzodiazepine family. Early trials of buspirone for nicotine withdrawal are encouraging.
Doxapram is a respiratory stimulant used for postoperative depression and alveolar hypoventilation syndromes. It stimulates peripheral chemoreceptors and brainstem respiratory centers. Dosage is 1-3 mg/mm by IV, up to a maximum of 600 mg. Doxapram can cause arrhythmias and hypertension by stimulating the release of epinephrine from the adrenals. It also can result in excessive CNS stimulation.
Progesterone is also a respiratory stimulant. It is used for Pickwickian syndrome. Progesterone is administered sublingually for outpatients. Inpatients are given a bolus of 100 mg/day IM. Progesterone takes 2-3 weeks for maximum effects to develop.
Naloxone is used to reverse ventilatory depression as a result of opiate administration (morphine, methadone, heroin). It is also effective in diazepam, propoxyphene, and ethanol overdoses. A bolus of 0.4-2.0 mg is given, but some patients may only require 0.1 mg/kg. Naloxone is short-acting so continuous infusion may be necessary.
Muscle relaxants and sedatives are used to improve the balance between gas exchange and the rate of metabolism. Muscle relaxants (paralyzers) must be used in combination with adequate sedation. If not, patients become extremely frightened and possibly psychotic. Indications for their use are shivering after bypass surgery, difficult intubations, and temporary control of the ventilator patient.
Pancuronium (Pavulon) is probably the most common paralyzer used. Intermittent bolus administration is recommended. If tachycardia develops from its use, Vecuronium should be substituted. Vecuronium can be given as a continuous infusion. Atracurium can also be used, but is shorter-acting and can cause histamine release.
Agitation causes catecholamine release, can produce auto-PEEP in ventilator patients, and causes an imbalance between O2 delivery and O2 consumption. Sedatives prevent these adverse conditions from occurring. Haloperidol (Haldol) is the preferred sedative because of its lack of ventilatory depression or hypotension. It can cause cardiovascular depression if hypovolemia is present or if given with propranolol. A 3-5 mg bolus is given IV and if there is no response in 15-20 minutes the dose is doubled. Another option if there is no response is to add a Ben Zodiazepine (Valium, Ativan, Versed). However, these can cause ventilatory depression, and can result in rapid sedation and accumulate in the body.
Morphine Sulfate (MS) is used for vasodilation, CNS sedation, and has a mild inotropic effect in patients with pulmonary edema. If the patient is not hypotensive, 5-10 mg of MS is given slowly via IV over several minutes. MS lowers pulmonary capillary pressure resulting in less leakage. Patient anxiety is also relieved. Ventilatory depression is possible but is rarely a problem.
Problems associated with MS are rapidly reversed with naloxone. One should avoid the use of MS as a sedative for asthmatic patients. MS (and other narcotics) cause histamine release and worsen asthma symptoms. Both oral and aerosolized MS have been used to increase exercise tolerance in the COPD patient. MS may reduce perceptions of dyspnea by acting directly on lung afferent nerves. This increases exercise capacity. An oral dose of 0.8 mg/kg or aerosol dose of 5 ml of a 1 mg/ml MS solution improve exercise endurance in these patients.
Propylene Glycol is a physiologically inert substance found in many aerosol preparations. It is used as a solvent and stabilizing agent. It is hygroscopic and used to minimize shrinkage of aerosol particles as they travel through the respiratory tract.
Pulmonary hypertension is defined as a mean pulmonary artery pressure >25 mm Hg at rest or >30 mm Hg with exercise. Most cases of pulmonary hypertension are secondary, meaning they are a result of another process. For example, hypoxia causes pulmonary vasoconstriction and therefore hypertension. Treatment for these causes of hypertension consist of fixing the primary problem, rather than treating the hypertension. “Primary” pulmonary hypertension is not a result of another problem. It is treated with a vasodilator or anticoagulants.
Primary pulmonary hypertension (PPH) usually develops in the third or fourth decade of life. Without treatment, most patients die within 2-3 years. PPH is a result of pulmonary capillary lumen cellular proliferation, thrombi, or fibrosis. Warfarin and Heparin have been used for anticoagulation and to prevent further thrombi. Vasodilators are effective for some patients, but not all. However, those who respond initially have a favorable response over the long term. Epoprostenol is given to test the patient’s response. If favorable, continue its use. The alternative is heart‑lung or single lung transplantation.
Respiratory pharmacology consists of medications used to treat the pathological triad of bronchospasm, airway inflammation, and retained secretions. Drugs used for these are bronchodilators, decongestants, corticosteroids, and mucokinetic/mucolytic agents. There are several routes of administration with the aerosol route being the most often used by the RCP. Some of the advantages of aerosol therapy include:
· rapid therapeutic effect
· a small total dose may be given
· topical administration minimizes systemic side-effects
The disadvantages of aerosol therapy consist of:
· underdosage or overdosage
· very little medication actually deposited in the lung
· airway irritation
· systemic absorption through oropharyngeal deposition
Excluding mainstream nebulization of large volumes of H2O, there are 4 methods of aerosol medication delivery. They are:
1. metered‑dose inhaler (MDI)
2. dry powder inhaler (DPI)
3. small volume nebulizer (SVN)
4. IPPB
The patient must be able to take a deep coordinated breath for the first three. IPPB is reserved for the patient who cannot spontaneously hyperinflate their lungs. Many conditions require modification of the recommended dose. Conditions such as liver dysfunction, kidney dysfunction, mechanical ventilation, emaciation, obesity, very young or very old patients require appropriate adjustment of dosage.
Bronchodilation is most often achieved through SNS stimulation. SNS stimulation results in conversion of ATP to cAMP through activation of the enzyme adenylate cyclase. This leads to airway smooth muscle relaxation. Beta adrenergic agonists increase the production of cAMP through the above mechanism. The enzyme phosphodiesterase inactivates cAMP thereby removing its bronchodilating influence.
Theophylline may work by inhibiting phosphodiesterase but this is unproven. Another mechanism to achieve bronchodilation is to block PNS stimulation of the airway. PNS stimulation leads to bronchoconstriction. Antimuscarinics are used to block PNS stimulation thereby leading to bronchodilation.
Complications of the Beta adrenergic agonists include tachycardia, skeletal muscle tremor and tachyphylaxis. Tachycardia is a result of excessive Beta1 stimulation. A worsening of V/Q relationships is also possible with these drugs. Beta2 stimulation can result in both bronchodilation and vasodilation. If the circulatory response exceeds the airway response, hypoxia may ensue. Complications of theophylline include tachycardia and tremors. It also can cause nausea and headache. Therapeutic levels should be maintained between 10-20 mcg/ml.
Decongestants and corticosteroids are not “bronchodilators” but their effects can lead to an increase in lumen size. Decongestants, through stimulation of receptors, cause vasoconstriction and decrease fluid in the airway. The resulting decrease in airway wall thickness and fluid in the lumen have the same effect as a bronchodilator.
Corticosteroids can have the same result through their anti-inflammatory properties. They also help prevent episodes of bronchospasm through inhibition of the allergic response to irritants. Chronic or excessive steroid use can result in adrenal insufficiency, cushioned effects, decreased resistance to infection and osteoporosis. Acute complications include fluid and electrolyte imbalances. If steroids prove successful, cromolyn sodium may be of benefit to the patient. It is a prophylactic drug only, preventing bronchospasm through inhibition of mast cell degranulation.
Mucokinetic/mucolytic drugs aid secretion removal by one or more of several mechanisms. They dilute mucus, replenish the sol layer, stimulate bronchial glands, stimulate cilia, or chemically destroy components found in mucus. The safest and most common mucokinetic is water. Normal saline solutions also are very common, particularly for lavage purposes. A common mucolytic is N-acetylcysteine. Its action is to rupture disulfide bonds of mucus making it less tenacious and viscous. Bronchospasm is a possible complication of its use. Complications of the mucokinetic/mucolytic agents are overhydration, bronchospasm and tissue irritation.
Antibiotics are rarely aerosolized. Several (carbenicillin, streptomycin, gentamicin) have been aerosolized with mixed results. Antibiotic lavage solutions are administered during a bronchoscopy for severe infections. Cystic fibrosis is a condition that may benefit from this. The penicillins and aminoglycosides are common antibiotics. Complications of various
antibiotics include hypersensitivity reactions, nausea, vomiting, diarrhea, inner ear problems, renal problems, and rarely, a blockade of the phrenic nerve.
Personalized medicine got one step closer to reality last week as the FDA issued its final guidelines on submission of pharmacogenomic data. “FDA’s efforts will bring us one step closer to ‘personalizing’ medical treatment,” according to prepared remarks by Janet Woodcock, the FDA’s deputy director of operations. “This new technology will allow medicines to be uniquely crafted to maximize their therapeutic benefits and minimize their potential risks for each patient.”
Indeed, this approach has already been used in the development of several targeted therapies – most notably Genentech Inc.’s Herceptin for metastatic breast cancer. But matching a patient's genetic profile with the best available drug is far from common practice today.
By focusing on pharmacogenomics, though, the FDA has clearly signaled its endorsement of this approach as a means to speed the development of new, targeted medicines.
The document, (http://www.fda.gov/cder/guidance/6400fnl.pdf) “Guidance For Industry: Pharmacogenomic Data Submissions,” clarifies how the agency will evaluate pharmacogenomic data – a critical and complicated issue that the FDA and industry spent considerable time discussing through a series of workshops over the last three years or so. These gatherings represented an unprecedented level of collaboration between the parties, and the resulting guidelines reflect a careful, thoughtful consideration of the issues involved from the perspective of each.
New Frontiers
The guidelines
describe what pharmacogenomic data companies will need to submit as part of the
regulatory review process, when to submit these data, the format for the
submissions and how the data will be used in regulatory decision making.
In particular, the guidelines differentiate various classes of biomarkers on which these tests are based. Very few established, validated, pharmacogenomic tests exist today: Only a smattering, primarily related to markers for drug metabolism such as CYP2D6 and thiopurine methyltransferase, are "well established biomarkers with clear clinical significance," according to the guidelines.
If a company has such data, then it is suggested that they be submitted with the IND.
That situation will change over time, of course, but for now the guidelines stipulate that “a pharmacogenomic test may be considered a valid biomarker if
1. it is measured in an analytical test system with well established performance characteristics and
2. there is an established scientific framework or body of evidence that elucidates the physiologic, pharmacologic, toxicologic, or clinical significance of the test results.”
While there are few known valid biomarkers, there are many more probable valid biomarkers, which “appear to have predictive value for clinical outcomes, but may not yet be widely accepted or independently verified by other investigators or institutions,” according to the guidelines.
If a company has data “sufficient to establish a significant association between a pharmacogenomic test result and clinical outcomes,” the guidelines suggest that a company submits these data with unapproved NDAs or BLAs.
Volunteers
Because the FDA
intends to play an active role in the evaluation of pharmacogenomic tests going
forward, it also wants to learn from research data as they are being generated
by biotech and pharmaceutical companies. To this end, the agency has created a
means by which companies can voluntarily submit research data without risking
the chance that they will be subject to regulatory review. Rather, they will be
used to help build and maintain expertise in this rapidly changing field.
That’s an assurance that many companies seem to need, for they've reportedly been wary of what the agency might do with these oft preliminary, exploratory data. The formal guidelines should help dispel those misgivings. And the agency has established a cross-center interdisciplinary pharmacogenomic review group (IPRG) to review these voluntary submissions.
As well, the FDA is encouraging companies to submit data from pharmacogenomic tests that have been used “to support drug development and/or guide therapy,"” such as a test that’s used to “stratify patients in a clinical trial or to identify patients at higher risk for an adverse event to correlate test results with clinical outcome.”
And, if a drug sponsor intends to include pharmacogenomic test data
in the drug's label “to choose a dose and dose schedule, to identify patients
at risk, or to identify patient responders,” the FDA recommends the
co-development of the drug and the test and submission of complete information
on the combination. (Further guidance on this issue is forthcoming, according
to the agency.)
Consensus
Thus, it will be no
easy task to come up with new, validated biomarkers. For this to occur, “both
the science and the technology have to be equally developed,” explained Dennis
Gilbert, chief scientific officer at Applied Biosystems
Inc.
For researchers, that means the scientific basis for any particular biomarker must be widely accepted in the research community and validated through peer reviewed publications. For companies like Applied Biosystems, which provides instrumentation, that means the machines must be standardized and give reproducible results. The same holds true for assays, of course, whatever their exact nature. Interestingly, the FDA has not defined any specific assay (say, PCR or microarray) as being the one of choice. Instead, that seems to be a function of the science and the application.
The guidelines also expand the opportunities for alliances between tool companies and drug developers, especially in sequencing, genotyping and gene expression, Gilbert added.
As well, tool providers can partner directly with the FDA: In early
March, Applied Biosystems entered a collaboration with the FDA's National
Center for Toxicological Research (NCTR) to study the toxicity profile of the
diabetes drug Rezulin via the firm's expression array system and rat genome
survey microarray. Rezulin (troglitazone) was withdrawn from the market in 2000
due to severe liver toxicity; the collaborators hope to determine the molecular
basis of this toxicity and see whether other insulin-sensitizing experimental
or marketed drugs of the same class elicit similar or dissimilar gene
expression profiles.
New
Perspectives
Pharmaceutical companies have been reluctant to jump wholeheartedly into so-called personalized medicine because they worry that the markets for new drugs will shrink as responding subpopulations are identified.
But given the current environment – in which both the agency and the drug industry are under fire on drug safety issues – the concept of developing less risky medicines targeted to known responders has apparently become more appealing.
Plus, the financial success of the newest batch of targeted cancer therapies provides proof that the markets can still be plenty attractive.
Perhaps one of the most important repercussions of the guidelines, however, is the fact that they remove regulatory uncertainty surrounding pharmacogenomic data submission. The guidelines provide drug sponsors with a sort of “how-to” manual backed by the FDA's assurance that it is wholly supportive of the use of pharmacogenomics in the development of new medicines.
| Oral versus Inhaled Asthma Therapy |