| Course |
· Diagnosis of COPD is based on a history of exposure to risk factors and the presence of airflow limitation that is not fully reversible, with or without the presence of symptoms. - Patients who have chronic cough and sputum production with a history of exposure to risk factors should be tested for airflow limitation, even if they do not have dyspnea.
· For the diagnosis and assessment of COPD, spirometry is the gold standard as it is the most reproducible, standardized, and objective way of measuring airflow limitation. FEV1/FVC < 70% and a post-bronchodilator FEV1 < 80% predicted confirms the presence of airflow limitation that is not fully reversible.
· Healthcare workers involved in the diagnosis and management of COPD patients should have access to spirometry.
· Measurement of arterial blood gas tensions should be considered in all patients with FEV1 < 40% predicted or clinical signs suggestive of respiratory failure or right heart failure.
A diagnosis of COPD should be considered in any patient who has cough, sputum production, or dyspnea, and/or a history of exposure to risk factors for the disease (Figure 5-1-1). The diagnosis is confirmed by spirometry. The presence of a post-bronchodilator FEV1 < 80% of the predicted value in combination with an FEV1/FVC < 70% confirms the presence of airflow limitation that is not fully reversible. Where spirometry is unavailable, the diagnosis of COPD should be made using all available tools. Clinical symptoms and signs, such as abnormal shortness of breath and increased forced expiratory time, can be used to help with the diagnosis. A low peak flow is consistent with COPD, but has poor specificity since it can be caused by other lung diseases and by poor performance. In the interest of improving the diagnosis of COPD, every effort should be made to provide access to standardized spirometry.
Although exceptions occur, the general patterns of symptom development in COPD are well established. The main symptoms among patients in Stage 0: At Risk and Stage I: Mild COPD are chronic cough and sputum production. These symptoms can be present for many years before the development of airflow limitation and are often ignored or discounted by patients. As airflow limitation develops in Stage II: Moderate COPD, patients often experience dyspnea, which may interfere with their daily activities. Typically, this is the stage at which they seek medical attention and are diagnosed with COPD. However, some patients do not experience cough, sputum production, or dyspnea in Stage I: Mild COPD or Stage II: Moderate COPD, and do not come to medical attention until their airflow limitation becomes more severe or their lung function is worsened acutely by a respiratory tract infection. As airflow limitation worsens and the patient enters Stage III: Severe COPD, the symptoms of cough and sputum production typically continue, dyspnea worsens, and additional symptoms heralding complications may develop. It is important to note that, since COPD may be diagnosed at any stage, any of the symptoms described below may be present in a patient presenting for the first time.
|
Table 5.1.1 - Key Indicators for Considering a Diagnosis of COPD |
|
|
|
Chronic cough: |
· Present intermittently or every day. Often present throughout the day; seldom only nocturnal. |
|
|
Chronic sputum production: |
· Any pattern of chronic sputum production may indicate COPD. |
|
|
Dyspnea that is: |
· Progressive (worsens over time). · Persistent (present every day). · Described by the patient as: “increased effort to breathe”, “heaviness”, “air hunger”, or “gasping”. · Worse on exercise. · Worse during respiratory infections. |
|
|
History of exposure to risk factors, especially: |
· Tobacco smoke · Occupational dusts and chemicals · Smoke from home cooking and heating fuels |
|
Consider COPD and perform spirometry if any of these indicators are present. These indicators are not diagnostic by themselves, but the presence of multiple key indicators increases the probability of a diagnosis of COPD. Spirometry is needed to establish a diagnosis of COPD.
Cough: Chronic cough, usually the first symptom of COPD to develop1, is often discounted by the patient as an expected consequence of smoking and/or environmental exposures. Initially, the cough may be intermittent, but later is present every day, often throughout the day, and is seldom entirely nocturnal. The chronic cough in COPD may be unproductive2. In some cases, significant airflow limitation may develop without the presence of a cough. Figure 5-1-2 lists some of the other causes of chronic cough in individuals with a normal chest X-ray.
Figure 5.1.2 Causes of Chronic Cough with a Normal Chest X-ray
|
Intrathoracic |
|
· Chronic Obstructive Pulmonary Disease · Bronchial Asthma · Central Bronchial Carcinoma · Endobronchial Tuberculosis · Bronchiectasis · Left Heart Failure · Interstitial Lung Disease · Cystic Fibrosis |
|
Extrathoracic |
|
· Postnasal Drip |
|
· Gastroesophageal Reflux |
|
· Drug Therapy (ACE inhibitors) |
Sputum production. COPD patients commonly raise small quantities of tenacious sputum after coughing bouts. Regular production of sputum for 3 or more months in 2 consecutive years is the epidemiological definition of chronic bronchitis3, but this is a somewhat arbitrary definition that does not reflect the range of sputum production in COPD patients. Sputum production is often difficult to evaluate because patients may swallow sputum rather than expectorate it, a habit subject to significant cultural and gender variation.
Dyspnea. Dyspnea, the hallmark symptom of COPD, is the reason most patients seek medical attention and is a major cause of disability and anxiety associated with the disease. Typical COPD patients describe their dyspnea as a sense of increased effort to breathe, heaviness, air hunger, or gasping4. The terms used to describe dyspnea vary both by individual and by culture5. It is often possible to distinguish the breathlessness of COPD from that due to other causes by analysis of the terms used, although there is considerable overlap with descriptors of bronchial asthma. A simple way to quantify the impact of breathlessness on a patient’s health status is the British Medical Research Council (MRC) questionnaire (Figure 5-1-3). This questionnaire relates well to other measures of health status6.
Breathlessness in COPD is characteristically persistent and progressive. Even on “good days” COPD patients experience dyspnea at lower levels of exercise than unaffected people of the same age. Initially, breathlessness is only noted on unusual effort (e.g., walking or running up a flight of stairs) and may be avoided entirely by appropriate behavioral change (e.g., using an elevator). As lung function deteriorates, breathlessness becomes more intrusive, and patients may notice that they are unable to walk at the same speed as other people of the same age or carry out activities that require use of the accessory respiratory muscles (e.g., carrying grocery bags)7. Eventually, breathlessness is present during everyday activities (e.g., dressing, washing) or at rest, leaving the patient confined to the home.
Wheezing and chest tightness. Wheezing and chest tightness are relatively non-specific symptoms that may vary between days, and over the course of a single day. These symptoms may be present in Stage I: Mild COPD, but are more characteristic of asthma or Stage III: Severe COPD. Audible wheeze may arise at a laryngeal level and need not be accompanied by auscultatory abnormalities. Alternatively, widespread inspiratory or expiratory wheezes can be present on listening to the chest. Chest tightness often follows exertion, is poorly localized, is muscular in character, and may arise from isometric contraction of the intercostal muscles. An absence of wheezing or chest tightness does not exclude a diagnosis of COPD.

Additional symptoms in severe disease. Weight loss and anorexia are common problems in advanced COPD8. Hemoptysis can occur during respiratory tract infections in COPD patients9. However, this can be a sign of other diseases (e.g., tuberculosis, bronchial tumors) and therefore should always be investigated. Cough syncope occurs due to rapid increases in intrathoracic pressure during attacks of coughing. Coughing spells may also cause rib fractures, which are sometimes asymptomatic. Psychiatric morbidity, especially symptoms of depression and/or anxiety, is common in advanced COPD10. Ankle swelling can be the only symptomatic pointer to the development of cor pulmonale.
· A detailed medical history of a new patient known or thought to have COPD should assess:
· Patient’s exposure to risk factors: such as smoking and occupational or environmental exposures.
· Past medical history: including asthma, allergy, sinusitis or nasal polyps, respiratory infections in childhood, other respiratory diseases.
· Family history of COPD or other chronic respiratory disease.
· Pattern of symptom development: COPD typically develops in adult life and most patients are conscious of increased breathlessness, more frequent “winter colds,” and some social restriction for a number of years before seeking medical help.
· History of exacerbations or previous hospitalizations for respiratory disorder: Patients may be aware of periodic worsening of symptoms even if these episodes have not been identified as acute exacerbations of COPD.
· Presence of co-morbidities: such as heart disease and rheumatic disease, which may also contribute to restriction of activity.
· Appropriateness of current medical treatments: For example, beta-blockers commonly prescribed for heart disease are usually contraindicated in COPD.
· Impact of disease on patient’s life: including limitation of activity; missed work and economic impact; effect on family routines; feelings of depression or anxiety.
· Social and family support available to the patient.
· Possibilities for reducing risk factors, especially smoking cessation.
Though an important part of patient care, a physical examination is rarely diagnostic in COPD. Physical signs of airflow limitation are usually not present until significant impairment of lung function has occurred11,12, and their detection has a relatively low sensitivity and specificity. A number of physical signs may be present in COPD, but their absence does not exclude the diagnosis.
· Central cyanosis, or bluish discoloration of the mucosal membranes, may be present but is difficult to detect in artificial light and in many racial groups.
· Common chest wall abnormalities, which reflect the pulmonary hyperinflation seen in COPD, include relatively horizontal ribs, “barrel- shaped” chest, and protruding abdomen.
· Flattening of the hemi-diaphragms may be associated with paradoxical in-drawing of the lower rib cage on inspiration, reduced cardiac dullness, and widening xiphisternal angle.
· Resting respiratory rate is often increased to more than 20 breaths per minute and breathing can be relatively shallow12.
· Patients commonly show pursed-lip breathing, which may serve to slow expiratory flow and permit more efficient lung emptying.
· COPD patients often have resting muscle activation while lying supine. Use of the scalene and sternocleidomastoid muscles is a further indicator of respiratory distress.
· Ankle or lower leg edema can be a sign of right heart failure.
· These are often unhelpful in COPD.
· Detection of the heart apex beat may be difficult due to pulmonary hyperinflation.
· Hyperinflation also leads to downward displacement of the liver and an increase in the ability to palpate this organ without it being enlarged.
· Patients with COPD often have reduced breath sounds, but this finding is not sufficiently characteristic to make the diagnosis13.
· The presence of wheezing during quiet breathing is a useful pointer to airflow limitation. However, wheezing heard only after forced expiration is of no diagnostic value.
· Inspiratory crackles occur in some COPD patients but are of little help diagnostically.
· Heart sounds are best heard over the xiphoid area. Measurement of Airflow Limitation (Spirometry)
Spirometry measurements should be undertaken for any patient who may have COPD. To help identify individuals earlier in the course of the disease, spirometry should be performed for patients who have chronic cough and sputum production even if they do not have dyspnea. Although spirometry does not fully capture the impact of COPD on a patient’s health, it remains the gold standard for diagnosing the disease and monitoring its progression. It is the best standardized, most reproducible, and most objective measurement of airflow limitation available. Healthcare workers who care for COPD patients should have access to spirometry, which is useful in both diagnosis and periodic monitoring. Figure 5-1-4 summarizes some considerations that are crucial to achieving consistently accurate test results.
Figure 5.1.4 Considerations in Performing Spirometry
· Spirometers need calibration on a regular basis.
· Spirometers should produce hard copy to permit detection of technical errors.
· The supervisor of the test needs training in its effective performance.
· Maximal patient effort in performing the text is required to avoid errors in diagnosis and management.
Performance
· Spirometry should be performed using techniques that meet published standards.
· The expiratory volume/time traces should be smooth and free from irregularities.
· The recording should go on long enough for a volume plateau to be reached, which may take more than 12 seconds in severe disease.
· Both FVC and FEV1 should be the largest value obtained from any of three technically satisfactory curves and the FVC and FEV1 values in these three curves should vary by no more than 5% or 100 ml, whichever is greater.
Evaluation
· Spirometry measurements are evaluated by comparison of the results with appropriate reference values based on age, height, sex and race.
· The presence of a postbronchodilator FEV1 <80% predicted together with an FEV1/FVC <70% confirms the presence of airflow limitation that is not fully reversible.
· In patients with FEV1 <80% predicted, FEV1/FVC <70% may be an early indicator of developing airflow limitation.
Spirometry should measure the maximal volume of air forcibly exhaled from the point of maximal inspiration (forced vital capacity, FVC) and the volume of air exhaled during the first second of this maneuver (forced expiratory volume in one second, FEV1), and the ratio of these two measurements (FEV1/FVC) should be calculated. Spirometry measurements are evaluated by comparison with reference values based on age, height, sex, and race (use appropriate reference values, e.g., see reference 14).
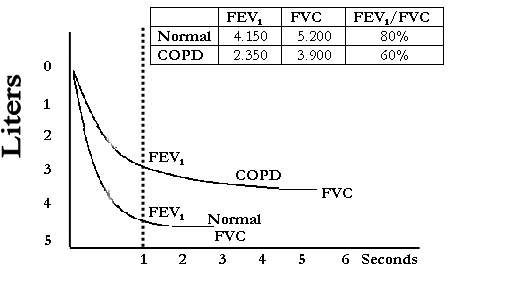 Figure 5-1-5
shows a normal spirogram and a spirogram typical of patients with mild to
moderate COPD. Patients with COPD typically show a decrease in both FEV1
and FVC. The degree of spirometric abnormality generally reflects the severity
of COPD (Figure 1-2). The presence of a post-bronchodilator FEV1
< 80% of the predicted value in combination with an FEV1/FVC <
70% confirms the presence of airflow limitation that is not fully reversible.
The FEV1/FVC on its own is a more sensitive measure of airflow
limitation, and an FEV1/FVC < 70% is considered an early sign of
airflow limitation in patients whose FEV1 remains normal (
Figure 5-1-5
shows a normal spirogram and a spirogram typical of patients with mild to
moderate COPD. Patients with COPD typically show a decrease in both FEV1
and FVC. The degree of spirometric abnormality generally reflects the severity
of COPD (Figure 1-2). The presence of a post-bronchodilator FEV1
< 80% of the predicted value in combination with an FEV1/FVC <
70% confirms the presence of airflow limitation that is not fully reversible.
The FEV1/FVC on its own is a more sensitive measure of airflow
limitation, and an FEV1/FVC < 70% is considered an early sign of
airflow limitation in patients whose FEV1 remains normal (![]() 80% predicted). This approach to defining airflow limitation is a pragmatic one
in view of the fact that universally applicable reference values for FEV1
and FVC are not available.
80% predicted). This approach to defining airflow limitation is a pragmatic one
in view of the fact that universally applicable reference values for FEV1
and FVC are not available.
Figure 5.1.5 Normal Spirogram and Spirogram Typical of Patients
with Mild and Moderate COPD
Peak expiratory flow (PEF) is sometimes used as a measure of airflow limitation, but in COPD the relationship between PEF and FEV1 is poor. PEF may underestimate the degree of airway obstruction in these patients15. If spirometry is unavailable, prolongation of the forced expiratory time beyond 6 seconds is a crude, but useful, guide to the presence of an FEV1/FVC ratio < 50%16,17.
The role of screening spirometry in the general population or in a population at risk for COPD is controversial. Both FEV1 and FVC predict all-cause mortality independent of tobacco smoking, and abnormal lung function identifies a subgroup of smokers at increased risk for lung cancer. This has been the basis of an argument that screening spirometry should be employed as a global health assessment tool18. However, there are no data to indicate that screening spirometry is effective in directing management decisions or in improving COPD outcomes.
Assessment of COPD severity is based on
the patient’s level of symptoms, the severity of the spirometric abnormality,
and the presence of complications such as respiratory failure and right heart
failure (Figure 1-2). The use of specific spirometric cut-points (e.g.,
FEV1![]() 80% predicted) to define different stages of COPD
is for purposes of simplicity; these cut-points have not been clinically
validated and may underestimate the prevalence of COPD in some groups, such as
the elderly.
80% predicted) to define different stages of COPD
is for purposes of simplicity; these cut-points have not been clinically
validated and may underestimate the prevalence of COPD in some groups, such as
the elderly.
Although the presence of airflow limitation is key to the current understanding of COPD, it may be valuable from a public health perspective to identify individuals at risk for the disease before significant airflow limitation develops (Stage 0, At Risk). A majority of people with early COPD identified in large studies complained of at least one respiratory symptom, such as cough, sputum production, wheezing, or breathlessness19,20. These symptoms may be present at a time of relatively minor or even no spirometric abnormality. While not all individuals with such symptoms will go on to develop COPD21, the presence of these symptoms should help define a high-risk population that should be targeted for preventive intervention. Much depends on the success of convincing such people, as well as healthcare workers, that minor respiratory symptoms may be markers of future ill health.
The severity of a patient’s breathlessness is important and can be gauged by the MRC scale (Figure 5-1-3). Arterial blood gases should be measured in all patients who have FEV1 < 40% predicted or clinical signs of respiratory failure or right heart failure.
For patients diagnosed with Stage II: Moderate COPD and beyond, the following additional investigations may be useful.
Bronchodilator reversibility testing. Generally performed only once, at the time of diagnosis, this test is useful for several reasons:
· To help rule out a diagnosis of asthma. If FEV1 returns to the predicted normal range after administration of a bronchodilator, the patient’s airflow limitation is likely due to asthma.
· To establish a patient’s best attainable lung function at that point in time.
· To gauge a patient’s prognosis. Some studies show that the post-bronchodilator FEV1 is a more reliable prognostic marker than pre-bronchodilator FEV122. In addition, the Intermittent Positive Pressure Breathing (IPPB) Study, a multicenter clinical trial, suggested that the degree of bronchodilator response is inversely related to the rate of FEV1 decline in COPD patients23.
· To assess potential response to treatment. Patients who show significant improvement in FEV1 after administration of a bronchodilator are more likely to benefit from treatment with bronchodilators and have a positive response to glucocorticosteroids. However, individual responses to bronchodilator tests are influenced by many factors, and failure of FEV1 to change by an arbitrary amount on one day does not preclude a response on another. Moreover, even patients who do not show a significant FEV1 response to a short-acting bronchodilator test may benefit symptomatically from long-term bronchodilator therapy.
Between-day reproducibility of spirometry in the same individual is approximately 178 ml24. Thus, an acute change that exceeds both 200 ml and 12% of the base line measurement is unlikely to have arisen by chance. A protocol for bronchodilator reversibility testing is listed in Figure 5-1-6.
|
Figure 5.1.6 Bronchodilator Reversibility Testing |
|
Preparation |
|
· Tests should be performed when patients are clinically stable and free from respiratory infection. · Patients should not have taken inhaled short-acting bronchodilators in the previous six hours, long acting b2 agonists in the previous 12 hours, or sustained release theophyllines in the previous 24 hours. |
|
Spirometry |
|
· FEV1 should be measured before a bronchodilator is given. · The bronchodilator should be given be metered dose inhaler through a spacer device or by nebulizer to be certain it has been inhaled. · The bronchodilator dose should be selected to be high on the dose/response curve. · Suitable dosage protocols are 400 mg b2 agonists, 80 mg anticholinergic, or the two combined. FEV1 should be measured again 30-45 minutes after the bronchodilator is given. |
|
Results |
|
· An increase in FEV1 that is both greater then 200 ml and 12% above the pre-bronchodilator FEV1 is considered significant. |
Glucocorticosteroid reversibility testing. Long-term glucocorticosteroid treatment in COPD can at present only be justified in patients with a consistent, significant FEV1 response to glucocorticosteroids, or in those with repeated exacerbations. The simplest, and potentially safest, way to identify these patients is by a treatment trial with inhaled glucocorticosteroids for 6 weeks to 3 months, using as criteria for glucocorticosteroid reversibility an FEV1 increase of 200 ml and 15% above baseline25,26. The response to glucocorticosteroids should be evaluated with respect to the post-bronchodilator FEV1 (i.e., the effect of treatment with inhaled glucocorticosteroids should be in addition to that of regular treatment with a bronchodilator). Where treatment with glucocorticosteroids is restricted for economic reasons to patients with a substantial spirometric response, a trial of oral glucocorticosteroid therapy may help select those with the largest response. However, prolonged oral glucocorticosteroid treatment beyond 2 weeks is NOT recommended in clinically stable patients.
Chest X-ray. A chest X-ray is seldom diagnostic in COPD unless obvious bullous disease is present, but it is valuable in excluding alternative diagnoses. Radiological changes associated with COPD include signs of hyperinflation (flattened diaphragm on the lateral chest film, and an increase in the volume of the retrosternal air space), hyperlucency of the lungs, and rapid tapering of the vascular markings. Computed tomography (CT) of the chest is not routinely recommended. However, when there is doubt about the diagnosis of COPD, high resolution CT (HRCT) might help in the differential diagnosis. In addition, if a surgical procedure such as bullectomy or lung volume reduction is contemplated, chest CT is helpful.
Arterial blood gas measurement. In advanced COPD measurement of arterial blood gases is important. This test should be performed in patients with FEV1 < 40% predicted or with clinical signs suggestive of respiratory failure or right heart failure.
Alpha-1 antitrypsin deficiency screening. In patients who develop COPD at a young age (< 45 years) or who have a strong family history of the disease, it may be valuable to identify coexisting alpha-1 antitrypsin deficiency. This could lead to family screening or appropriate counseling. A serum concentration of alpha-1 antitrypsin below 15-20 % of the normal value is highly suggestive of homozygous alpha-1 antitrypsin deficiency.
A major differential diagnosis is asthma. In some patients with chronic asthma, a clear distinction from COPD is not possible using current imaging and physiological testing techniques, and it is assumed that asthma and COPD coexist in these patients. In these cases, current management is similar to that of asthma. Other potential diagnoses are usually easier to distinguish from COPD.
Visits to healthcare facilities will increase in frequency as COPD progresses. The type of healthcare workers seen, and the frequency of visits, will depend on the healthcare system. Ongoing monitoring and assessment in COPD ensures that the goals of treatment are being met and should include evaluation of:
1. exposure to risk factors, especially tobacco smoke
2. disease progression and development of complications
3. pharmacotherapy and other medical treatment
4. exacerbation history
5. co-morbidities.
Suggested questions for follow-up visits are listed in Figure 5-1-8. The best way to detect changes in symptoms and overall health status is to ask the same questions at each visit.
Figure 5-1-8 Suggested Questions for Follow-up Visits
Monitor exposure risk factors:
· Have you continued to stay off cigarettes?
· If not, how many cigarettes per day are you smoking?
· Would you like to quit smoking?
· Has there been any change in your working environment?
Monitor disease progression and development of complications:
· How much can you do before you get short of breath? (Use an everyday example as walking up flights of stairs, up a hill, or on flat ground.)
· Has your dyspnea worsened, improved, or stayed the same since your last visit?
· Have you had to reduce your activities because of dypsnea or other symptoms?
· Have any of your symptoms worsened since your last visit?
· Have you experienced any new symptoms since your last visit?
· Has your sleep been disrupted due to dyspnea or other symptoms?
· Since your last visit, have you missed any work because of your symptoms?
Monitor pharmacotherapy and other medical treatment:
· What medications are you taking?
· How often so you take each medication?
· How much do you take each time?
· Have you missed or stopped taking any regular doses of your medications for any reason?
· Have you had trouble filling your prescriptions? (e.g. for financial reasons, not on formulary)
· Please show me how you use your inhaler.
· Have you tried any other medications or remedies?
· Has your medication been effective in controlling your symptoms?
· Has your medication caused you any problems?
Monitor exacerbation history:
· Since your last visit, have you had any episodes/times when your symptoms were a lot worse then usual?
· If so, how long did the episode(s) last? What do you think caused the symptoms to get worse? What did you do to control the symptoms?
COPD is usually a progressive disease. Lung function can be expected to worsen over time, even with the best available care. Symptoms and objective measures of airflow limitation should be monitored to determine when to modify therapy and to identify any complications that may develop. As at the initial assessment, follow-up visits should include a physical examination and discussion of symptoms, particularly any new or worsening symptoms.
Pulmonary function. A patient’s decline in lung function is best tracked by periodic spirometry measurements. Useful information about lung function decline is unlikely from spirometry measurements performed more than once a year. Spirometry should be performed if there is a substantial increase in symptoms or a complication.
Other pulmonary function tests, such as flow-volume loops, diffusing capacity (DLCO) measurements, and measurement of lung volumes are not needed in a routine assessment but can provide information about the overall impact of the disease and can be valuable in resolving diagnostic uncertainties and assessing patients for surgery.
Arterial blood gas measurement. Measurement of arterial blood gas tensions should be performed in all patients with FEV1 < 40% predicted or when clinical signs of respiratory failure or right heart failure are present. Respiratory failure is indicated by a PaO2 < 8.0 kPa (60 mm Hg) with or without PaCO2 > 6.0 kPa (45 mm Hg) in arterial blood gas measurements made while breathing air at sea level.
Screening patients by pulse oximetry and assessing arterial blood gases in those with an oxygen saturation (SaO2) < 92% may be a useful way of selecting patients for arterial blood gas measurement27. However, pulse oximetry gives no information about CO2 tensions.
Several considerations are important to ensure accurate test results. Oxygen pressure in the inspired air (FiO2) should be measured, taking note if patient is using an O2-driven nebulizer. Changes in arterial blood gas tensions take time to occur, especially in severe disease. Thus, 20-30 minutes should pass before rechecking the gas tensions when the FiO2 has been changed.
Adequate pressure must be applied at the puncture site for at least one minute; failure to do so can lead to painful bruising.
Clinical signs of respiratory failure or right heart failure include central cyanosis, ankle swelling, and an increase in the jugular venous pressure. Clinical signs of hypercapnia are extremely nonspecific outside of acute exacerbations.
Assessment of pulmonary hemodynamics. Pulmonary hypertension is only likely to be important in patients who have developed respiratory failure. Measurement of pulmonary arterial pressure is not recommended in clinical practice as it does not add practical information beyond that obtained from a knowledge of PaO2.
Diagnosis of right heart failure or cor pulmonale. Elevation of the jugular venous pressure and the presence of pitting ankle edema are often the most useful findings suggestive of cor pulmonale in clinical practice. However, the jugular venous pressure is often difficult to assess in patients with COPD, due to large swings in intrathoracic pressure. Firm diagnosis of cor pulmonale can be made through a number of investigations, including radiography, electrocardiography, echocardiography, radionucleotide scintigraphy, and magnetic resonance imaging. However, all of these measures involve inherent inaccuracies of diagnosis.
CT and ventilation-perfusion scanning. Despite the benefits of being able to delineate pathological anatomy, routine CT and ventilation-perfusion scanning are currently confined to the assessment of COPD patients for surgery. HRCT is currently under investigation as a way of visualizing airway and parenchymal pathology more precisely.
Hematocrit. Polycythemia can develop in the presence of arterial hypoxemia, especially in continuing smokers28. Polycythemia can be identified by hematocrit > 55%.
Respiratory muscle function. Respiratory muscle function is usually measured by recording the maximum inspiratory and expiratory mouth pressures. More complex measurements are confined to research laboratories. Measurement of expiratory muscle force is useful in assessing patients when dyspnea or hypercapnia is not readily explained by lung function testing or when peripheral muscle weakness is suspected. This measurement may improve in COPD patients when other measurements of lung mechanics do not (e.g., after pulmonary rehabilitation)29,30.
Sleep studies. Sleep studies may be indicated when hypoxemia or right heart failure develops in the presence of relatively mild airflow limitation or when the patient has symptoms suggesting the presence of sleep apnea.
Exercise testing. Several types of tests are available to measure exercise capacity, but these are primarily used in conjunction with pulmonary rehabilitation programs.
In order to adjust therapy appropriately as the disease progresses, each follow-up visit should include a discussion of the current therapeutic regimen. Dosages of various medications, adherence to the regimen, inhaler technique, effectiveness of the current regime at controlling symptoms, and side effects of treatment should be monitored.
During periodic assessments, healthcare workers should question the patient and evaluate any records of exacerbations, both self-treated and those treated by other healthcare providers. Frequency, severity, and likely causes of exacerbations should be evaluated. Increased sputum volume, acutely worsening dyspnea, and the presence of purulent sputum should be noted. Specific inquiry into unscheduled visits to providers, telephone calls for assistance, and use of urgent or emergency care facilities may be helpful. Severity can be estimated by the increased need for bronchodilator medication or glucocorticosteroids and by the need for antibiotic treatment. Hospitalizations should be documented, including the facility, duration of stay, and any use of critical care or intubation. The clinician then can request summaries of all care received to facilitate continuity of care.
In treating patients with COPD, it is important to consider the presence of concomitant conditions such as bronchial carcinoma, tuberculosis, sleep apnea, and left heart failure. The appropriate diagnostic tools (chest radiograph, ECG, etc.) should be used whenever symptoms (e.g., hemoptysis) suggest one of these conditions.
1. Georgopoulos D, Anthonisen NR. Symptoms and signs of COPD. In: Cherniack NS, ed. Chronic obstructive pulmonary disease. Toronto: WB Saunders; 1991. p. 357-63.
2. Burrows B, Niden AH, Barclay WR, Kasik JE. Chronic obstructive lung disease II. Relationships of clinical and physiological findings to the severity of airways obstruction. Am Rev Respir Dis 1965; 91:665-78.
3. Medical Research Council. Definition and classification of chronic bronchitis for clinical and epidemiological purposes: a report to the Medical Research Council by their Committee on the etiology of Chronic Bronchitis. Lancet 1965; 1: 775-80.
4. Simon PM, Schwartstein RM, Weiss JW, Fencl V, Teghtsoonian M, Weinberger SE. Distinguishable types of dyspnea in patients with shortness of breath. Am Rev Respir Dis 1990; 142:1009-14.
5. Elliott MW, Adams L, Cockcroft A, MacRae KD, Murphy K, Guz A. The language of breathlessness. Use of verbal descriptors by patients with cardiopulmonary disease. Am Rev Respir Dis 1991; 144:826-32.
6. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581-6.
7. Celli BR, Rassulo J, Make BJ. Dyssynchronous breathing during arm but not leg exercise in patients with chronic airflow obstruction. N Engl J Med 1986; 314:1485-90.
8. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147:1151-6.
9. Johnston RN, Lockhart W, Ritchie RT, Smith DH. Haemoptysis. BMJ 1960; 1:592-5.
10. Calverley PM. Neuropsychological deficits in chronic obstructive pulmonary disease. Monaldi Archives for Chest Disease 1996; 51:5-6.
11. Kesten S, Chapman KR. Physician perceptions and management of COPD. Chest 1993; 104:254-8.
12. Loveridge B, West P, Kryger MH, Anthonisen NR. Alteration of breathing pattern with progression of chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 134:930-4.
13. Badgett RC, Tanaka DV, Hunt DK, Jelley MJ, Feinberg LE, Steiner JF, et al. Can moderate chronic obstructive pulmonary disease be diagnosed by history and physical findings alone? Am J Med 1993; 94:188-96.
14. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107-36.
15. Kelly CA, Gibson GJ. Relation between FEV1 and peak expiratory flow in patients with chronic obstructive pulmonary disease. Thorax 1988; 43:335-6.
16. Lal S, Ferguson AD, Campbell EJM. Forced expiratory time; a simple test for airways obstruction. BMJ 1964; 1:814-7.
17. Swanney MP, Jensen RL, Crichton DA, Beckert LE, Cardno LA, Crapo RO. FEV(6) is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000; 162:917-9.
18. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the national lung health education program. Chest 2000; 117:1146-61.
19. Kanner RE, Connett JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med 1999; 106:410-6.
20. Lofdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respir Med 1998; 92:467-72.
21. Peto R, Speizer FE, Cochrane AL, Moore F, fletcher CM, Tinker CM, et al. The relevance in adults of airflow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease: results from twenty years of prospective observation. Am Rev Respir Dis 1983; 128:491-500.
22. Hansen EF, Phanareth K, Laursen LC, KokJensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:1267-71.
23. Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133:814-9.
24. Sourk RL, Nugent KM. Bronchodilator testing: confidence intervals derived from placebo inhalations. Am Rev Respir Dis 1983; 128:153-7.
25. Reis AL. Response to bronchodilators. In: Clausen J, ed. Pulmonary function testing: guidelines and controversies. New York: Academic Press; 1982. p. 215-221.
26. American Thoracic Society-Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991; 144:1202-18.
27. Roberts CM, Bugler JR, Melchor R, Hetzel ML, Spiro SG. Value of pulse oximetry for long-term oxygen therapy requirement. Eur Respir J 1993; 6:559-62.
28. Calverley PM, Leggett RJ, McElderry L, Flenley DC. Cigarette smoking and secondary polycythemia in hypoxic cor pulmonale. Am Rev Respir Dis 1982; 125:507-10.
29. Dekhuijzen PNR, Folgering HT, van Herwaarden CLA. Target-flow inspiratory muscle training during pulmonary rehabilitation in patients with COPD. Chest 1991; 99:128-33.
30. Heijdra YF, Dekhuijzen PN, van Herwaarden CLA, Forlgering H. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. Am J Respir Crit Care Med 1996; 153:260-5.
| Course |