| Course |
· Reduction of total personal exposure to tobacco smoke, occupational dusts and chemicals, and indoor and outdoor air pollutants are important goals to prevent the onset and progression of COPD.
· Smoking cessation is the single most effective - and cost effective - way in most people to reduce the risk of developing COPD and stop its progression (Evidence A).
· Brief tobacco dependence counseling is effective (Evidence A) and every tobacco user should be offered at least this treatment at every visit to a healthcare provider.
· Three types of counseling are especially effective: practical counseling, social support as part of treatment, and social support arranged outside of treatment (Evidence A).
· Several effective pharmacotherapies for tobacco dependence are available (Evidence A), and at least one of these medications should be added to counseling if necessary and in the absence of contraindications (Evidence A).
· Progression of many occupationally induced respiratory disorders can be reduced or controlled through a variety of strategies aimed at reducing the burden of inhaled particles and gases (Evidence B).
Identification, reduction, and control of risk factors are important steps toward prevention and treatment of any disease. In the case of COPD, these factors include tobacco smoke, occupational exposures, and indoor and outdoor air pollution and irritants.
Since cigarette smoking is the major risk factor for COPD worldwide, smoking prevention programs should be implemented and smoking cessation programs should be readily available and encouraged for all individuals who smoke. Reduction of total personal exposure to occupational dust, fumes, and gases and to indoor and outdoor air pollutants is also an important goal to prevent the onset and progression of COPD1.
Comprehensive tobacco control policies and programs with clear, consistent, and repeated nonsmoking messages should be delivered through every feasible channel, including healthcare providers, schools, and radio, television, and print media. National and local campaigns should be undertaken to reduce exposure to tobacco smoke in public forums. Legislation to establish smoke-free schools, public facilities, and work environments should be encouraged by government officials, public health workers, and the public. Smoking prevention programs should target all ages, including young children, adolescents, young adults, and pregnant women. Physicians and public health officials should encourage smoke-free homes.
The first exposure to cigarette smoke may begin in utero when the fetus is exposed to blood-borne metabolites from the mother2,3. Neonates and infants may be exposed passively to tobacco smoke in the home if a family member smokes. Children less than 2 years old who are passively exposed to cigarette smoke have an increased prevalence of respiratory infections, and are at a greater risk of developing chronic respiratory symptoms later in life4.
Smoking cessation is the single most effective - and cost effective - way to reduce exposure to COPD risk factors. Quitting smoking can prevent or delay the development of airflow limitation or reduce its progression5. A statement by the WHO (Figure 5-2-1)6 emphasizes the health and economic benefits to be gained from smoking cessation. All smokers - including those who may be at risk for COPD as well as those who already have the disease - should be offered the most intensive smoking cessation intervention feasible.
Figure 5.2.1
World Health Organization Statement on Smoking Cessation6
Smoking cessation is a critical step toward substantially reducing the health risks run by current smokers, thereby improving world health. Tobacco has been shown to cause about twenty-five life-threatening diseases, or groups of diseases, many of which can be prevented, delayed, or mitigated by smoking cessation. As life expectancy increases in developing countries, the morbidity and mortality burden of chronic diseases will increase still further. This projected concentration of tobacco-related disease burden can be lightened by intensive efforts at smoking cessation. Studies have shown that 75-80% of smokers want to quit, while one-third have made at least three serious cessation attempts. Cessation efforts cannot be ignored in favor of primary prevention; rather, both efforts must be made in conjunction with one another. If only small portions of today's 1.1 billion smokers were able to stop, the long-term health and economic benefits would be immense.
Governments, communities, organizations, schools, families and individuals are called upon to help current smokers stop their addictive and damaging habit. 9
Smoking cessation interventions are effective in both genders, in all racial and ethnic groups, and in pregnant women. Age influences quit rates, with young people less likely to quit, but nevertheless smoking cessation programs can be effective in all age groups.
International data on the economic impact of smoking cessation are strikingly consistent: investing resources in smoking cessation programs is cost effective in terms of medical costs per life year gained. Interventions that have been investigated include nicotine replacement with transdermal patch, counseling from physicians and other health professionals (with and without nicotine patch), self-help and group programs, and community-based stop-smoking contests. A review of data from a number of countries estimated the median societal cost of various smoking cessation interventions at $990 to $13,000 (US) per life year gained7. Smoking cessation programs are a particularly good value for the UK National Health Service, with costs from £212 to £873 (US $320 to $1,400) per life year gained8.
The role of healthcare providers in smoking cessation. A successful smoking cessation strategy requires a multifaceted approach, including public policy, information dissemination programs, and health education through the media and schools9. However, healthcare providers, including physicians, nurses, dentists, psychologists, pharmacists, and others, are key to the delivery of smoking cessation messages and interventions. Involving as many of these individuals as possible will help. Healthcare workers should encourage all patients who smoke to quit, even those patients who come to the healthcare provider for unrelated reasons and do not have symptoms of COPD or evidence of airflow limitation.
Guidelines for smoking cessation entitled Treating Tobacco Use and Dependence: A Clinical Practice Guideline were published by the US Public Health Service10. The major conclusions are summarized in Figure 5-2-2.
Figure 5-2-2 Public Health Service Report: Treating Tobacco Use and Dependence: A Clinical Practice Guideline - Major Findings and Recornmendations10
1. Tobacco dependence is a chronic condition that warrants repeated treatment until long-term or permanent abstinence is achieved.
2. Effective treatments for tobacco dependence exist and all tobacco users should be offered these treatments.
3. Clinicians and healthcare delivery systems must institutionalize the consistent identification, documentation and treatment of every tobacco user at every visit.
4. Brief tobacco dependence treatment is effective and every tobacco user should be offered at least brief treatment.
5. There is a strong dose- response relation between the intensity of tobacco dependence counseling and its effectiveness.
6. Three types of counseling were found to be especially effective: practical counseling, social support as part of treatment, and social support arranged outside of treatment.
7. Five first-line pharmacotherapies for tobacco dependence - bupropion SR, nicotine gum, nicotine inhaler, nicotine nasal spray, and nicotine patch - are effective and at least one of these medications should be prescribed in the absence of contraindications.
8. Tobacco dependence treatments are cost effective relative to other medical and disease prevention interventions.
The Public Health Service Guidelines recommend a five-step program for intervention (Figure 5-2-3), which provides a strategic framework helpful to healthcare providers interested in helping their patients stop smoking10-13. The guidelines emphasize that tobacco dependence is a chronic disease (Figure 5-2-4)10 and urge clinicians to recognize that relapse is common and reflects the chronic nature of dependence, not failure on the part of the clinician or the patient.
Table 5.2.3Brief Strategies to Help the Patient Willing to Quit Smoking78 |
|
1.
ASK: Systematically identify all
tobacco users at every visit.
2.
ADVISE: Strongly urge all tobacco
users to quit.
3.
ASSESS: Determine willingness to make
a quit attempt.
4.
ASSIST: Aid the patient in quitting.
5.
ARRANGE: Schedule follow-up contact.
|
Figure 5-2.4. Tobacco Dependenceas a Chronic Diseasel2
· For most people, tobacco dependence results in true drug dependence comparable to dependence caused by opiates, amphetamines, and cocaine.
· Tobacco dependence is almost always a chronic disorder that warrants long-term clinical intervention as do other addictive disorders. Failure to appreciate the chronic nature of tobacco dependence may impair the clinician’s motivation to treat tobacco use consistently in a long-term fashion.
· Clinicians must understand that this is a chronic condition comparable to diabetes, hypertension, or hyperlipidernia requiring simple counseling advice, support, and appropriate pharmacotherapy.
· Relapse is common, which is the nature of dependence and not the failure of the clinician or the patient.
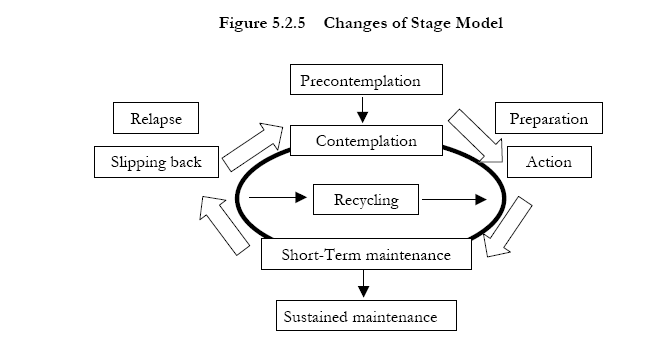
Most individuals go through several stages before they stop smoking (Figure 5-2-5)9. It is often helpful for the clinician to assess a patient’s readiness to quit in order to determine the most effective course of action at that time. The clinician should initiate treatment if the patient is ready to quit. For a patient not ready to make a quit attempt, the clinician should provide a brief intervention designed to promote the motivation to quit.
Counseling. Counseling delivered by physicians and other health professionals significantly increases quit rates over self-initiated strategies14. Even a brief (3-minute) period of counseling to urge a smoker to quit results in smoking cessation rates of 5-10%15. At the very least, this should be done for every smoker at every healthcare provider visit15,16.
However, there is a strong dose-response relationship between counseling intensity and cessation success17,18. Ways to make the treatment more intense include increasing the length of the treatment session, the number of treatment sessions, and the number of weeks over which the treatment is delivered. Counseling sessions of 3 to 10 minutes result in cessation rates of around 12%10. With more complex interventions (for example, controlled clinical trials that include skills training, problem solving, and psychosocial support), quit rates can reach 20-30%17. In one multicenter controlled clinical trial, a combination of physician advice, group support, skills training, and nicotine replacement therapy achieved a quit rate of 35% at one year and a sustained quit rate of 22% at 5 years5.
Both individual and group counseling are effective formats for smoking cessation. Several particular items of counseling content seem to be especially effective, including problem solving, general skills training, and provision of intra-treatment support. The important elements in the support aspect of successful treatment programs are shown in Figure 5-2-69,10. The common subjects covered in successful problem solving/skills training programs include:
· Recognition of danger signals likely to be associated with the risk of relapse, such as being around other smokers, being under time pressure, getting into an argument, drinking alcohol, and negative moods.
· Enhancement of skills needed to handle these situations, such as learning to anticipate and avoid a particular stress.
· Basic information about smoking and successful quitting, such as the nature and time course of withdrawal, the addictive nature of smoking, and the fact that any return to smoking, including even a single puff, increases the likelihood of a relapse.
Figure 5-2-6. Patient Support in Smoking Cessation Program
· Encourage the patient in the quit attempt.
Indicate that effective cessation treatments are now available and, in fact, half of all people who smoked have now quit. Communicate your confidence in the patient's ability to quit.
· Communicate care and concern.
Ask how the patient feels about smoking and whether he/she wants to quit, expressing concern along with the ability and willingness to help. Be open to the patient's fears of quitting.
· Encourage the patient to talk about the quitting process.
Talk to the patient about the reason she/she wants to quit, difficulty encountered while quitting, success the patient has achieved, and concerns and worries about quitting.
· Provide basic information about smoking, the risks of continuing, the benefits of quitting, and the techniques that optimize success.
Outline the nature, symptoms, and time course of withdrawal and techniques for dealing with withdrawal.
Pharmacotherapy. Numerous effective pharmacotherapies for smoking cessation now exist9-11 (Evidence A), and pharmacotherapy is recommended when counseling is not sufficient to help patients quit smoking. Special consideration should be given before using pharmacotherapy in selected populations: people with medical contraindications, light smokers (fewer than 10 cigarettes/day), and pregnant and adolescent smokers.
Nicotine replacement products. Numerous studies indicate that nicotine replacement therapy in any form (nicotine gum, inhaler, nasal spray, transdermal patch, sublingual tablet, or lozenge) reliably increases long-term smoking abstinence rates10,19. Nicotine replacement therapy is more effective when combined with counseling and behavior therapy20, although nicotine patch or nicotine gum consistently increases smoking cessation rates regardless of the level of additional behavioral or psychosocial interventions. Medical contraindications to nicotine replacement therapy include unstable coronary artery disease, untreated peptic ulcer disease, and recent myocardial infarction or stroke9. Specific studies to date do not support the use of nicotine replacement therapy for longer than eight weeks21, although some patients may require extended use to prevent relapse.
All forms of nicotine replacement therapy are significantly more effective than placebo. Every effort should be made to tailor the choice of replacement therapy to the individual’s culture and lifestyle to improve adherence. The patch is generally favored over the gum because it requires less training for effective use and is associated with fewer compliance problems.
No data are available to help clinicians tailor nicotine patch regimens to the intensity of cigarette smoking. In all cases it seems generally appropriate to start with the higher dose patch. For most patches, which come in three different doses, patients should use the highest dose for the first four weeks and drop to progressively lower doses over an eight-week period. Where only two doses are available, the higher dose should be used for the first four weeks and the lower dose for the second four weeks.
When using nicotine gum, the patient needs to be advised that absorption occurs through the buccal mucosa. For this reason, the patient should be advised to chew the gum for a while and then put the gum against the inside of the cheek to allow absorption to occur and prolong the release of nicotine. Continuous chewing produces secretions that are swallowed, results in little absorption, and can cause nausea. Acidic beverages, particularly coffee, juices, and soft drinks, interfere with the absorption of nicotine. Thus, the patient needs to be advised that eating or drinking anything except water should be avoided for 15 minutes before and during chewing. Although nicotine gum is an effective smoking cessation treatment, problems with compliance, ease of use, social acceptability, risk of developing temporomandibular joint symptoms, and unpleasant taste have been noted. In highly dependent smokers, the 4 mg gum is more effective than the 2 mg gum22.
Other pharmacotherapy. The antidepressants bupropion and nortriptyline have also been shown to increase long-term quit rates9,19,23. Although more studies need to be conducted with these medications, a randomized controlled trial with counseling and support showed quit rates at one year of 30% with sustained-release bupropion alone and 35% with sustained-release bupropion plus nicotine patch23. The effectiveness of the antihypertensive drug clonidine is limited by side effects19.
Although it is not known how many individuals are at risk of developing respiratory disease from occupational exposures in either developing or developed countries, many occupationally induced respiratory disorders can be reduced or controlled through a variety of strategies aimed at reducing the burden of inhaled particles and gases24:
· Implement and enforce strict, legally mandated control of airborne exposure in the workplace.
· Initiate intensive and continuing education of exposed workers, industrial managers, healthcare workers, primary care physicians, and legislators.
· Educate workers and policy-makers on how cigarette smoking aggravates occupational lung diseases and why efforts to reduce smoking where a hazard exists are important.
The main emphasis should be on primary prevention, which is best achieved by the elimination or reduction of exposures to various substances in the workplace. Secondary prevention, achieved through surveillance and early case detection, is also of great importance. Both approaches are necessary to improve the present situation and to reduce the burden of lung disease.
Individuals experience diverse indoor and outdoor environments throughout the day, each of which has its own unique set of air contaminants. Although outdoor and indoor air pollution are generally thought of separately, the concept of total personal exposure may be more relevant for COPD. Reducing the risk from indoor and outdoor air pollution requires a combination of public policy and protective steps taken by individual patients.
At the national level, achieving a set level of air quality should be a high priority; this goal will normally require legislative action. Details on setting and maintaining air quality goals are beyond the scope of this document.
Understanding health risks posed by local air pollution sources may be difficult and requires skills in community health, toxicology, and epidemiology. Local physicians may become involved through concerns about the health of their patients or as advocates for the community’s environment.
· The healthcare provider should consider susceptibility (including family history and exposure to indoor/outdoor pollution) for each individual patient.
· Patients should be counseled concerning the nature and degree of their susceptibility. Those who are at high risk should avoid vigorous exercise outdoors during pollution episodes.
· If various solid fuels are used for cooking and heating, adequate ventilation should be encouraged.
· Persons with severe COPD should monitor public announcements of air quality and should stay indoors when air quality is poor.
· The use of medication should follow the usual clinical indications; therapeutic regimes should not be adjusted because of the occurrence of a pollution episode without evidence of worsening of symptoms or function.
· Respiratory protective equipment has been developed for use in the workplace in order to minimize exposure to toxic gases and particles. However, under most circumstances, healthcare providers should not suggest respiratory protection as a method for reducing the risks of ambient air pollution.
· Air cleaners have not been shown to have health benefits, whether directed at pollutants generated by indoor sources or at those brought in with outdoor air.
1. Samet J, Utell MJ. Ambient air pollution. In: Rosenstock L, Cullen M, eds. Textbook of occupational and environmental medicine. Philadelphia: WB Saunders; 1994. p. 53-60.
2. Jeffery PK. Cigarette smoke-induced damage of airway mucosa. In: Chretien J, Dusser D, eds. Environmental impact on the airways: from injury to repair. Lung biology in health and disease. Vol. 93. New York: Marcel Dekker;1996. p. 299-354.
3. Helms PJ. Lung growth: implications for the development of disease [editorial]. Thorax 1994; 49:440-1.
4. Colley JR, Holland WW, Corkhill RT. Influence of passive smoking and parental phlegm on pneumonia and bronchitis in early childhood. Lancet 1974; 2:1031-4.
5. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272:1497-505.
6. World Health Organization. Tobacco free initiative: policies for public health. Geneva: World Health Organization; 1999. Available from: URL: www.who/int/toh/worldnottobacco99
7. Tengs TO, Adams ME, Pliskin JS, Safran DG, Siegel JE, Weinstein MC, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal 1995; 15:369-90.
8. Parrott S, Godfrey C, Raw M, West R, McNeill A. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Health Educational Authority. Thorax 1998; 53 (Suppl 5 Pt 2): S1-38.
9. Fiore MC, Bailey WC, Cohen SJ. Smoking cessation: information for specialists. Rockville, MD: US Department of Health and Human Services, Public Health Service, Agency for HealthcarePolicy and Research and Centers for Disease Control and Prevention; 1996. AHCPR Publication No. 96-0694.
10. The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. A clinical practice guideline for treating tobacco use and dependence. JAMA 2000; 28:3244-54.
11. American Medical Association. Guidelines for the diagnosis and treatment of nicotine dependence: how to help patients stop smoking. Washington, DC: American Medical Association; 1994.
12. Glynn TJ, Manley MW. How to help your patients stop smoking. A National Cancer Institute manual for physicians. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1990. NIH Publication No. 90-3064.
13. Glynn TJ, Manley MW, Pechacek TF. Physician-initiated smoking cessation program: the National Cancer Institute trials. Prog Clin Biol Res 1990; 339:11-25.
14. Baillie AJ, Mattick RP, Hall W, Webster P. Meta-analytic review of the efficacy of smoking cessation interventions. Drug and Alcohol Review 1994; 13:157-70.
15. Wilson DH, Wakefield MA, Steven ID, Rohrsheim RA, Esterman AJ, Graham NM. “Sick of smoking”: evaluation of a targeted minimal smoking cessation intervention in general practice. Med J Aust 1990; 152:518-21.
16. Britton J, Knox A. Helping people to stop smoking: the new smoking cessation guidelines [editorial]. Thorax 1999; 54:1-2.
17. Kottke TE, Battista RN, DeFriese GH, Brekke ML. Attributes of successful smoking cessation interventions in medical practice. A meta-analysis of 39 controlled trials.JAMA 1988; 259:2883-9.
18. Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician-delivered smoking interventions: a randomized clinical trial. J Gen Intern Med 1991; 6:1-8.
19. Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ 2000; 321:355-8.
20. Schwartz JL. Review and evaluation of smoking cessation methods: United States and Canada, 1978-1985. Bethesda, MD: National Institutes of Health; 1987. NIH Publication No. 87-2940.
21. Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. JAMA 1994; 271:1940-7.
22. Sachs DP, Benowitz NL. Individualizing medical treatment for tobacco dependence [editorial; comment]. Eur Respir J 1996; 9:629-31.
23. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999; 340:685-91.
24. The COPD Guidelines Group of the Standards of Care Committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52 Suppl 5:S1-28.
| Course |