| Course |
· The overall approach to managing stable COPD should be characterized by a stepwise increase in treatment, depending on the severity of the disease.
· For patients with COPD, health education can play a role in improving skills, ability to cope with illness, and health status. It is effective in accomplishing certain goals, including smoking cessation (Evidence A).
· None of the existing medications for COPD has been shown to modify the long-term decline in lung function that is the hallmark of this disease (Evidence A). Therefore, pharmacotherapy for COPD is used to decrease symptoms and/or complications.
· Bronchodilator medications are central to the symptomatic management of COPD (Evidence A). They are given on an as-needed basis or on a regular basis to prevent or reduce symptoms.
· The principal bronchodilator treatments are ß2-agonists, anticholinergics, theophylline, and a combination of these drugs (Evidence A).
· Regular treatment with inhaled glucocorticosteroids should only be prescribed for symptomatic COPD patients with a documented spirometric response to glucocorticosteroids or in those with an FEV1 < 50% predicted and repeated exacerbations requiring treatment with antibiotics or oral glucocorticosteroids (Evidence B).
· Chronic treatment with systemic glucocorticosteroids should be avoided because of an unfavorable benefit-to-risk ratio (Evidence A).
· All COPD patients benefit from exercise training programs, improving with respect to both exercise tolerance and symptoms of dyspnea and fatigue (Evidence A).
· The long-term administration of oxygen (> 15 hours per day) to patients with chronic respiratory failure has been shown to increase survival (Evidence A).
The overall approach to managing stable COPD should be characterized by a stepwise increase in treatment, depending on the severity of the disease. The step-down approach used in the chronic treatment of asthma is not applicable to COPD since COPD is usually stable and very often progressive. Management of COPD involves several objectives (see Chapter 5, Introduction) that should be met with minimal side effects from treatment. It is based on an individualized assessment of disease severity (Figure 5-3-1) and response to various therapies.
The classification of severity (Figure 1-2) of stable COPD incorporates an individualized assessment of disease severity and therapeutic response into the management strategy. This classification is a guide that should help healthcare workers make decisions about the management of COPD in individual patients. Treatment depends on the patient’s educational level and willingness to apply the recommended management, on cultural and local conditions, and on the availability of medications.
Figure 5-3-1. Factors Affecting the Severity of COPD
· Severity of symptoms
· Severity of airflow limitation
· Frequency and severity of exacerbations
· Presence of one or more complications
· Presence of respiratory failure
· Presence of comorbid conditions
· General health status
· Number of medications needed to manage the disease
Although patient education is generally regarded as an essential component of care for any chronic disease, the role of education in COPD has been poorly studied. Assessment of the value of education in COPD may be difficult because of the relatively long time required to achieve improvements in objective measurements of lung function.
Studies that have been done indicate that patient education alone does not improve exercise performance or lung function1-4 (Evidence B), but it can play a role in improving skills, ability to cope with illness, and health status5. These outcomes are not traditionally measured in clinical trials, but they may be most important in COPD where even pharmacologic interventions generally confer only a small benefit in terms of lung function.
Patient education regarding smoking cessation has the greatest capacity to influence the natural history of COPD. Evaluation of the smoking cessation component in a long-term, multicenter study indicates that if effective resources and time are dedicated to smoking cessation, 25% long-term quit rates can be maintained6 (Evidence A). Education also improves patient response to acute exacerbations7,8 (Evidence B). Prospective end-of-life discussions can lead to understanding of advance directives and effective therapeutic decisions at the end of life9 (Evidence B).
Ideally, educational messages should be incorporated into all aspects of care for COPD and may take place in many settings: consultations with physicians or other healthcare workers, home-care or outreach programs, and comprehensive pulmonary rehabilitation programs.
It is vital for patients with COPD to understand the nature of their disease, risk factors for progression, and their role and the role of healthcare workers in achieving optimal management and health outcomes. Education should be tailored to the needs and environment of the individual patient, interactive, directed at improving quality of life, simple to follow, practical, and appropriate to the intellectual and social skills of the patient and the caregivers.
In managing COPD, open communication between patient and physician is essential. In addition to being empathic, attentive and communicative, health professionals should pay attention to patients’ fears and apprehensions, focus on educational goals, tailor treatment regimens to each individual patient, anticipate the effect of functional decline, and optimize patients’ practical skills.
Several specific education strategies have been shown to improve patient adherence to medication and management regimens. In COPD, adherence does not simply refer to whether patients take their medication appropriately. It also covers a range of non-pharmacologic treatments - e.g., maintaining an exercise program after pulmonary rehabilitation, undertaking and sustaining smoking cessation, and using devices such as nebulizers, spacers, and oxygen concentrators properly.
The topics that seem most appropriate for an education program include: smoking cessation; basic information about COPD and pathophysiology of the disease; general approach to therapy and specific aspects of medical treatment; self-management skills; strategies to help minimize dyspnea advice about when to seek help; self-management and decision-making during exacerbations; and advance directives and end-of-life issues (Figure 5-3-2).
Education should be part of consultations with healthcare workers beginning at the time of first assessment for COPD and continuing with each follow-up visit. The intensity and content of these educational messages should vary depending on the severity of the patient’s disease. In practice, a patient often poses a series of questions to the physician. It is important to answer these questions fully and clearly, as this may help make treatment more effective.
Figure 5 -3-2. Topics for Patient Education
Stage 0: At Risk
· Information and advice about reducing risk factors
Stage I.- Mild COPD to Stage II Moderate COPD
Above topic, plus:
· Information about the nature of COPD
· Instruction on how to use inhalers and other treatments
· Recognition and treatment of acute exacerbations
· Strategies for minimizing dyspnea
Stage III: Severe COPD
Above topics, plus:
· Information about complications
· Information about oxygen treatment
· Advance directives and end-of-life decisions
There are several different types of educational programs, ranging from simple distribution of printed materials, to teaching sessions designed to convey information about COPD, to workshops designed to train patients in specific skills (e.g., self-management, peak flow monitoring).
Although printed materials may be a useful adjunct to other educational messages, passive dissemination of printed materials alone does not improve skills or health outcomes. Education is most effective when it is interactive and conducted in small workshops4 (Evidence B) designed to improve both knowledge and skills. Behavioral approaches such as cognitive therapy and behavior modification lead to more effective self-management skills and maintenance of exercise programs.
The cost effectiveness of education programs for COPD patients is highly dependent on local factors that influence the cost of access to medical services and that will vary substantially between countries. In one cost-benefit analysis of education provided to hospital inpatients with COPD10, an information package resulted in increased knowledge of COPD and reduced use of health services, including reductions of hospital readmissions and general practice consultations. The education package involved training patients to increase knowledge of COPD, medication usage, precautions for exacerbations, and peak flow monitoring technique. However, this study was undertaken in a heterogeneous group of patients - 65% were smokers and 88% were judged to have an asthmatic component to their disease - and these findings may not hold true for a “pure” COPD population.
Pharmacologic therapy is used to prevent and control symptoms, reduce the frequency and severity of exacerbations, improve health status, and improve exercise tolerance. None of the existing medications for COPD has been shown to modify the long-term decline in lung function that is the hallmark of this disease6,11,12,13 (Evidence A). However, this should not preclude efforts to use medications to control symptoms. Since COPD is usually progressive, recommendations for the pharmacological treatment of COPD reflect the following general principles:
· There should be a stepwise increase in treatment, depending on the severity of the disease. (The step-down approach used in the chronic treatment of asthma is not applicable to COPD.)
· Regular treatment needs to be maintained at the same level for long periods of time unless significant side effects occur or the disease worsens.
· Treatment response of an individual patient is variable and should be monitored closely and adjusted frequently.
The medications are presented in the order in which they would normally be introduced in patient care, based on the level of disease severity. However, each treatment regimen needs to be patient-specific as the relationship between the severity of symptoms and the severity of airflow limitation is influenced by other factors, such as the frequency and severity of exacerbations, the presence of one or more complications, the presence of respiratory failure, co-morbidities (cardiovascular disease, sleep-related disorders, etc.), and general health status.
Medications that increase the FEV1 or change other spirometric variables, usually by altering airway smooth muscle tone, are termed bronchodilators14, since the improvements in expiratory flow reflect widening of the airways rather than changes in lung elastic recoil. Such drugs improve emptying of the lungs, tend to reduce dynamic hyperinflation at rest and during exercise15, and improve exercise performance. The extent of these changes, especially in moderate to severe disease, is not easily predictable from the improvement in
FEV116,17. Regular bronchodilation with drugs that act primarily on airway smooth muscle does not modify the decline of function in mild COPD and, by inference, the prognosis of the disease6 (Evidence B).
Bronchodilator medications are central to the symptomatic management of COPD18-21 (Evidence A). They are given either on an as-needed basis for relief of persistent or worsening symptoms, or on a regular basis to prevent or reduce symptoms. The side effects of bronchodilator therapy are pharmacologically predictable and dose dependent. Adverse effects are less likely, and resolve more rapidly after treatment withdrawal, with inhaled than with oral treatment. However, COPD patients tend to be older than asthma patients and more likely to have co-morbidities, so their risk of developing side effects is greater. A summary of bronchodilator therapy in COPD is provided in Figure 5-3-4.
|
Figure 5.3.4 - Bronchodilators in Stable COPD |
|
· Bronchodilator medications are central to symptom management in COPD. · Inhaled therapy is preferred. · The choice between ß2-agonist, anticholinergic, theophylline, or combination therapy depends on availability and individual response in terms of symptom relief and side effects. · Bronchodilators are prescribed on an as-needed or on a regular basis to prevent or reduce symptoms. · Long-acting inhaled bronchodilators are more convenient. · Combining bronchodilators may improve efficacy and decrease the risk of side effects compared to increasing the dose of a single bronchodilator. |
When treatment is given by the inhaled route, attention to effective drug delivery and training in inhaler technique is essential. COPD patients may have more problems in effective coordination and find it harder to use a simple Metered Dose Inhaler (MDI) than do healthy volunteers or younger asthmatics. It is essential to ensure that inhaler technique is correct and to re-check this at each visit.
Alternative breath-activated or spacer devices are available for most formulations. Dry powder inhalers may be more convenient and possibly provide improved drug deposition, although this has not been established in COPD. In general, particle deposition will tend to be more central with the fixed airflow limitation and lower inspiratory flow rates in COPD22,23.
Wet nebulizers are not recommended for regular treatment because they are more expensive and require appropriate maintenance. A list of currently available inhaler devices is provided at http://www.goldcopd. com/inhalers/. The choice will depend on availability, cost, the prescribing physician, and the skills and ability of the patient.
Dose-response relationships using the FEV1 as the outcome are relatively flat with all classes of bronchodilators18-21. The dose-response relationships for anticholinergics and ß2-agonists are shown in Figure 5-3-5 21. Toxicity is also dose related. Increasing the dose of either a ß2-agonist or an anticholinergic by an order of magnitude, especially when given by a wet nebulizer, appears to provide subjective benefit in acute episodes24 (Evidence B) but is not necessarily helpful in stable disease25 (Evidence C).
Figure 5.3.5 Dose-Response Relationships for Bronchodilators
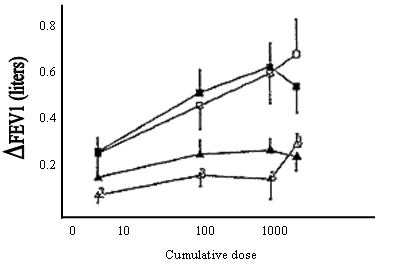
Open symbols=salbutamol (ß2-agonists)
Closed symbols-ipratropium (anticholinergic)
Squares=patients with asthma
Triangles=patients with COPD
Inhaled ß2-agonists have a relatively rapid onset of bronchodilator effect although this is probably slower in COPD than in asthma. The bronchodilator effects of short-acting ß2-agonists usually wear off within 4 to 6 hours26,27 (Evidence A). Long-acting inhaled ß2-agonists, such as salmeterol and formoterol, show a duration of effect of 12 hours or more with no loss of effectiveness overnight or with regular use in COPD patients28-30 (Evidence A).
All categories of bronchodilators have been shown to increase exercise capacity in COPD, without necessarily producing significant changes in FEV125,31,32 (Evidence A). Regular treatment with short-acting bronchodilators is cheaper but less convenient than treatment with long-acting bronchodilators. In doses of 50 µg twice daily, but not 100 µg twice daily33 (Evidence B), the long-acting inhaled ß2-agonist salmeterol has been shown to improve health status significantly. Similar data for short-acting ß2-agonists are not available. Use of inhaled ipratropium (an anticholinergic) four times daily also improves health status34 (Evidence B). Theophylline is effective in COPD, but due to its potential toxicity inhaled bronchodilators are preferred when available. All studies that have shown efficacy of theophylline in COPD were done with slow-release preparations. The classes of bronchodilator drugs commonly used in treating COPD, ß2-agonists, anticholinergics, and methylxanthines, are shown in Figure 5-3-6. The choice depends on the availability of medication and the patient’s response.
Figure 5.3.6Commonly Used Formulations of Bronchodilator Drugs |
|||||
|
Druga |
Metered Dose Inhaler (µg)b |
Nebulizer (mg)b |
Oral (mg)b |
Duration of Action (hrs) |
|
|
ß2-agonists |
- |
- |
- |
- |
|
|
Fenoterol |
100-200 |
0.5-2.0 |
– |
4-6 |
|
|
Salbutamol (albuterol)c |
100-200 |
2.5-5.0 |
4 |
4-6 |
|
|
Terbutaline |
250-500 |
5-10 |
5 |
4-6 |
|
|
Formoterol |
12-24 |
– |
- |
12+ |
|
|
Salmeterol |
50-100 |
– |
– |
12+ |
|
|
Anticholinergics |
- |
- |
- |
- |
|
|
Ipratropium bromide |
40-80 |
0.25-0.5 |
– |
6-8 |
|
|
Oxitropium bromide |
200 |
– |
– |
7-9 |
|
|
Methylxanthinesd |
– |
– |
– |
– |
|
|
Aminophylline (SR) |
– |
– |
225-450 |
Variable, up to 24 |
|
|
Theophylline (SR) |
– |
– |
100-400 |
Variable, up to 24 |
|
|
a. Not all products are available in all countries. b. Doses: ß2-agonists refer to average dose given up to 4 times daily for short-acting and 2 times daily for long-acting preparations; anticholinergics are usually given 3-4 times daily. c. Name in parentheses refers to North American generic term. d. Methylxanthines require dose titration depending on side effects and plasma theophylline levels. |
|||||
ß2-agonists. The principal action of ß2-agonists is to relax airway smooth muscle by stimulating ß2-adrenergic receptors, which increases cyclic AMP and produces functional antagonism to bronchoconstriction. Oral therapy is slower in onset and has more side effects than inhaled treatment35 (Evidence A).
Adverse effects. Stimulation of ß2-receptors can produce resting sinus tachycardia and has the potential to precipitate cardiac rhythm disturbances in very susceptible patients, although this appears to be a remarkably rare event with inhaled therapy. Exaggerated somatic tremor is troublesome in some older patients treated with higher doses of ß2-agonists, whatever the route of administration, and this limits the dose that can be tolerated.
Although hypokalemia can occur, especially when treatment is combined with thiazide diuretics36, and oxygen consumption can be increased under resting conditions37, these metabolic effects show tachyphylaxis unlike the bronchodilator actions. Mild falls in PaO2 occur after administration of both short- and long-acting ß2-agonists38, but the clinical significance of these changes is doubtful. Despite the concerns raised some years ago, further detailed study has found no association between ß2-agonist use and an accelerated loss of lung function or increased mortality in COPD.
Anticholinergics. The most important effect of anticholinergic medications in COPD patients appears to be blockage of acetylcholine’s effect on M3 receptors. Current drugs also block M2 receptors and modify transmission at the pre-ganglionic junction, although these effects appear less important in COPD39.
The bronchodilating effect of short-acting inhaled anticholinergics lasts longer than that of short-acting ß2-agonists, with some bronchodilator effect generally apparent up to 8 hours after administration26 (Evidence A).
Adverse effects. Anticholinergic drugs, such as ipratropium bromide, are poorly absorbed, which limits the troublesome systemic effects seen with atropine. Extensive use of this class of inhaled agents in a wide range of doses and clinical settings has shown them to be very safe. Although occasional prostatic symptoms have been reported, there are no data to prove a true causal relationship. A bitter, metallic taste is reported by some patients using ipratropium.
Use of wet nebulizer solutions with a face mask has been reported to precipitate acute glaucoma, probably by a direct effect of the solution on the eye. Mucociliary clearance is unaffected by these drugs, and respiratory infection rates are not increased.
Methylxanthines. Controversy remains about the exact effects of xanthine derivatives. They may act as non-selective phosphodiesterase inhibitors, but have also been reported to have a range of non-bronchodilator actions, the significance of which is disputed31,40-44. Data on duration of action for conventional, or even slow-release, xanthine preparations are lacking in COPD. Changes in inspiratory muscle function have been reported in patients treated with theophylline40, but whether this reflects changes in dynamic lung volumes or a primary effect on the muscle is not clear (Evidence B). All studies that have shown efficacy of theophylline in COPD were done with slow-release preparations. Theophylline is effective in COPD but, due to its potential toxicity, inhaled bronchodilators are preferred when available.
Adverse effects. Toxicity is dose related, a particular problem with the xanthine derivatives because their therapeutic ratio is small and most of the benefit occurs only when near-toxic doses are given42,43 (Evidence A). Methylxanthines are nonspecific inhibitors of all phosphodiesterase enzyme subsets, which explains their wide range of toxic effects. Problems include the development of atrial and ventricular arrhythmias (which can prove fatal) and grand mal convulsions (which can occur irrespective of prior epileptic history). More common and less dramatic side effects include headaches, insomnia, nausea, and heartburn, and these may occur within the therapeutic range of serum theophylline. Unlike the other bronchodilator classes, xanthine derivatives may involve a risk of overdose (either intentional or accidental).
Theophylline, the most commonly used methylxanthine, is metabolized by cytochrome P450 mixed function oxidases. Clearance of the drug declines with age. Many other physiological variables and drugs modify theophylline metabolism; some of the potentially important interactions are listed in Figure 5-3-7.
|
Figure 5-3-7 Drugs and Physiological Variables that Affect Theophylline Metabolism in COPD |
|
|
Increased |
Decreased |
|
· Tobacco smoking · Anticonvulsant drugs · Rifampicin · Alcohol |
· Old age · Arterial hypoxemia• (Pa02< 6.0 kPa, 45• mm Hg) · Respiratory acidosis · Congestive cardiac failure · Liver cirrhosis · Erythromycin · Quinolone antibiotics · Cimetidine (not ranitidine) · Viral infections · Herbal remedies (St.John's Wort) |
Combination therapy. Combining drugs with different mechanisms and durations of action may increase the degree of bronchodilation for equivalent or lesser side effects. A combination of a short-acting ß2-agonist and the anticholinergic drug ipratropium in stable COPD produces greater and more sustained improvements in FEV1 than either drug alone and does not produce evidence of tachyphylaxis over 90 days of treatment26,45,46 (Evidence A).
The combination of a ß2-agonist, an anticholinergic, and/or theophylline may produce additional improvements in lung function26,28,44,47 and health status26,28,42,48. Increasing the number of drugs usually increases costs, and an equivalent benefit may occur by increasing the dose of one bronchodilator when side effects are not a limiting factor. Detailed assessments of this approach have not been carried out.
Bronchodilator therapy by disease severity. Figure 5-3-8 provides a summary of bronchodilator and other treatment at each stage of COPD. For patients with few or intermittent symptoms (Stage I: Mild COPD), short-acting inhaled therapy as needed to control dyspnea or coughing spasms is sufficient. If inhaled bronchodilators are not available, regular treatment with slow-release theophylline should be considered. ß2-agonists and anticholinergics taken by inhalation are generally equipotent49, with some studies suggesting that the latter are more likely to be effective in a given clinical setting7 (Evidence A). Consideration of costs and possible side effects will determine the choice of drug for monotherapy, but for patients with Stage I: Mild COPD or Stage II: Moderate COPD as-needed treatment with either is a reasonable first step. Failure of one of these bronchodilator classes to control symptoms should prompt a trial of the other class, and if symptoms are still troublesome, regular treatment with a combination of drugs is appropriate. One post-hoc review has suggested that hospitalization days are reduced in patients whose treatment regimens contain an inhaled anticholinergic50 (Evidence C), but this issue requires prospective study as it would be of considerable economic importance if confirmed. One-time, objective changes in spirometry are a poor guide to the long-term, subjective benefit of bronchodilator treatment. Empirical treatment trials, rather than a laboratory assessment of bronchodilator response, should be used to determine whether treatment should continue.
|
Figure 5.3.8 Therapy at Each Stage of COPD |
|||
|
Patients must be taught how and when to use their treatments and treatments being prescribed for other conditions should be reviewed. Beta-blocking agents (including eye drop formulations) should be avoided. |
|||
|
Stage |
Characteristics |
Recommended treatment |
|
|
ALL |
|
· Avoidance of risk factor(s) · Influenza vaccination |
|
|
0: At Risk |
· Chronic symptoms (cough, sputum) · Exposure to risk factor(s) · Normal spirometry |
|
|
|
I: Mild COPD |
· FEV1/FVC<70% · FEV1³80% predicted · With or without symptoms |
Short-acting bronchodilator when needed |
|
|
II: Moderate COPD |
IIA: · FEV1/FVC<70% · 50%˜FEV1<80% predicted · with or without symptoms |
· Regular treatment with one or more bronchodilators · Rehabilitation |
Inhaled glucocorticosteroids if significant symptoms and lung function response |
|
IIB: · FEV1/FVC<70% · 30%˜FEV1<50% predicted · with or without symptoms |
· Regular treatment with one or more bronchodilator · Rehabilitation |
Inhaled glucocorticosteroids if significant symptoms and lung function response or if repeated exacerbations |
|
|
III: Severe COPD |
· FEV1/FVC<70% · FEV1<30% predicted or presence of respiratory failure or right heart failure |
· Regular treatment with one or more bronchodilators · Inhaled glucocorticosteroids if significant symptoms and lung function response or if repeated exacerbations · Treatment of complications · Rehabilitation · Long-term oxygen therapy if respiratory failure · Consider surgical treatments |
|
Patients with Stage II: Moderate COPD to Stage III: Severe COPD who are on regular short- or long-acting bronchodilator therapy may also use a short-acting bronchodilator as needed.
For patients who remain highly symptomatic, the addition of oral slow-release theophylline can be tried, but it should be titrated against directly measured plasma theophylline levels to reduce the risk of serious side effects and obtain maximum benefit. Some patients may request regular treatment with high-dose nebulized bronchodilators51, especially if they have experienced subjective benefit from this treatment during an acute exacerbation. Clear scientific evidence for this approach is lacking, but one option is to examine the improvement in mean daily peak expiratory flow recording during two weeks of treatment in the home and continue with nebulizer therapy if a significant change occurs51. In general, nebulized therapy for a stable patient is not appropriate unless it has been shown to be better than conventional dose therapy.
The effects of oral and inhaled glucocorticosteroids in COPD are much less dramatic than in asthma, and their role in the management of stable COPD is limited to very specific indications. The use of glucocorticosteroids for the treatment of acute exacerbations is described in Component 4: Manage Exacerbations.
Oral glucocorticosteroids - short-term. Many existing COPD guidelines recommend the use of a short course (two weeks) of oral glucocorticosteroids to identify COPD patients who might benefit from long-term treatment with oral or inhaled glucocorticosteroids. This recommendation is based on evidence52 that short-term effects predict long-term effects of oral glucocorticosteroids on FEV1, and evidence that asthma patients with airflow limitation might not respond acutely to an inhaled bronchodilator but do show significant bronchodilation after a short course of oral glucocorticosteroids.
There is mounting evidence, however, that a short course of oral glucocorticosteroids is a poor predictor of the long-term response to inhaled glucocorticosteroids in COPD13,53. For this reason, there appears to be insufficient evidence to recommend a therapeutic trial with oral glucocorticosteroids in patients with Stage II: Moderate COPD or Stage III: Severe COPD and poor response to an inhaled bronchodilator.
Oral glucocorticosteroids - long-term. Two retrospective studies54,55 analyzed the effects of treatment with oral glucocorticosteroids on long-term FEV1 changes in clinic populations of patients with moderate to severe COPD. The retrospective nature of these studies, the lack of a true control group, and the imprecise definition of COPD are reasons for a cautious interpretation of the data and conclusions.
A side effect of long-term treatment with systemic glucocorticosteroids is steroid myopathy56-58, which contributes to muscle weakness, decreased functionality, and respiratory failure in subjects with advanced COPD. In view of the well-known toxicity of long-term treatment with oral glucocorticosteroids, it is not surprising that no prospective studies have been performed on the long-term effects of these drugs in COPD.
Therefore, based on the lack of evidence of benefit, and the large body of evidence on side effects, long-term treatment with oral glucocorticosteroids is not recommended in COPD (Evidence A).
Inhaled glucocorticosteroids. Many studies have looked at the short-term effect of inhaled glucocorticosteroids on pulmonary function parameters in COPD. While some studies have shown a significant improvement, others have not59-72. The major problems with most studies are the small number of subjects and the short duration of treatment. However, data from four large studies on the long-term effects of inhaled glucocorticosteroids in COPD (Copenhagen City12, EUROSCOP11, ISOLDE13, Lung Health Study II73) provide evidence that regular treatment with inhaled glucocorticosteroids is only appropriate for symptomatic COPD patients with a documented spirometric response to inhaled glucocortico-steroids (see Component 1: Assess and Monitor Disease) or in those with an FEV1 < 50% predicted (Stage IIB: Moderate COPD and Stage III: Severe COPD) and repeated exacerbations requiring treatment with antibiotics or oral glucocorticosteroids (Evidence B).
The dose-response relationships and long-term safety of inhaled glucocorticosteroids in COPD are not known. Only moderate to high doses have been used in long-term clinical trials. Two studies showed an increased incidence of skin bruising in a small percentage of the COPD patients11,13. One long-term study showed no effect of budesonide on bone density and fracture rate11, while another study showed that treatment with triamcinolone acetamide was associated with a decrease in bone density73. The efficacy and side effects of inhaled glucocorticosteroids in asthma are dependent on the dose and type of glucocorticosteroid74. This pattern can also be expected in COPD and needs documentation in this patient population. Treatment with inhaled glucocorticosteroids can be recommended for patients with more advanced COPD and repeated acute exacerbations as described in Component 4: Manage Exacerbations.
Vaccines. Influenza vaccines can reduce serious illness and death in COPD patients by about 50%75 (Evidence A). Vaccines containing killed or live, inactivated viruses are recommended76 as they are more effective in elderly patients with COPD77. The strains are adjusted each year for appropriate effectiveness and should be given once (in Autumn) or twice (in Autumn and Winter) each year. A pneumococcal vaccine containing 23 virulent serotypes has been used, but sufficient data to support its general use in COPD patients are lacking78-80 (Evidence B).
Alpha-1 antitrypsin augmentation therapy. Young patients with severe hereditary alpha-1 antitrypsin deficiency and established emphysema may be candidates for alpha-1 antitrypsin augmentation therapy. However, this therapy is very expensive, is not available in most countries, and is not recommended for patients with COPD that is unrelated to alpha-1 antitrypsin deficiency (Evidence C).
Antibiotics. In several large-scale controlled studies81-83, prophylactic, continuous use of antibiotics was shown to have no effect on the frequency of acute exacerbations in COPD. Another study examined the efficacy of winter chemoprophylaxis over a period of 5 years and concluded that there was no benefit84. Thus, on the present evidence, the use of antibiotics, other than for treating infectious exacerbations of COPD and other bacterial infections, is not recommended85,86 (Evidence A).
Mucolytic (mucokinetic, mucoregulator) agents (ambroxol, erdosteine, carbocysteine, iodinated glycerol). The regular use of mucolytics in COPD has been evaluated in a number of long-term studies with controversial results87-89. The majority showed no effect of mucolytics on lung function or symptoms, although some have reported a reduction in the frequency of acute exacerbations. A Cochrane collaborative review performed a meta-analysis of all the available data, including that from a number of abstracts90. A statistically significant reduction in the number of episodes of chronic bronchitis was found in patients treated with mucolytics compared to those receiving placebo. However, these data are not easy to interpret, as the follow-up ranged from 2 to 6 months and the patients all had an FEV1 > 50% predicted. Although a few patients with viscous sputum may benefit from mucolytics91,92, the overall benefits seem to be very small. Therefore, the widespread use of these agents cannot be recommended on the basis of the present evidence (Evidence D).
Antioxidant agents. Antioxidants, in particular N-acetylcysteine, have been shown to reduce the frequency of exacerbations and could have a role in the treatment of patients with recurrent exacerbations93-96 (Evidence B). However, before their routine use can be recommended, the results of ongoing trials will have to be carefully evaluated.
Immunoregulators (immunostimulators, immunomodulators). A study using an immunostimulator in COPD showed a decrease in the severity (though not in the frequency) of exacerbations97, but these results have not been duplicated. Thus, the regular use of this therapy cannot be recommended based on the present evidence98 (Evidence B).
Antitussives. Cough, although sometimes a troublesome symptom in COPD, has a significant protective role99. Thus the regular use of antitussives is contraindicated in stable COPD (Evidence D).
Vasodilators. The belief that pulmonary hypertension in COPD is associated with a poorer prognosis has provoked many attempts to reduce right ventricular afterload, increase cardiac output, and improve oxygen delivery and tissue oxygenation. Many agents have been evaluated, including inhaled nitric oxide, but the results have been uniformly disappointing. In patients with COPD, in whom hypoxemia is caused primarily by ventilation-perfusion mismatching rather than by increased intrapulmonary shunt (as in noncardiogenic pulmonary edema), inhaled nitric oxide can worsen gas exchange because of altered hypoxic regulation of ventilation-perfusion balance100,101. Therefore, based on the available evidence, nitric oxide is contraindicated in stable COPD.
Respiratory stimulants. Almitrine bismesylate, a relatively specific peripheral chemoreceptor stimulant that increases ventilation at any level of CO2 under hypoxemic conditions, has been studied in both stable respiratory failure and acute exacerbations. It improves ventilation-perfusion relationships by modifying the hypoxic vasoconstrictor response. Oral almitrine has been shown to improve oxygenation, but to a lesser degree than low doses of inspired O2. There is no evidence that almitrine improves survival or quality of life, and in large clinical trials it was associated with a number of significant side effects, particularly peripheral neuropathy102-104. Therefore, on the present evidence almitrine is not recommended for regular use in stable COPD patients (Evidence B). The use of doxapram, a non-specific but relatively safe respiratory stimulant available as an intravenous formulation, is not recommended in stable COPD (Evidence D).
Narcotics (morphine). Narcotics are contraindicated in COPD because of their respiratory depressant effects and potential to worsen hypercapnia. Clinical studies suggest that morphine used to control dyspnea may have serious adverse effects and its benefits may be limited to a few sensitive subjects105-109. Codeine and other narcotic analgesics should also be avoided.
Others. Nedocromil, leukotriene modifiers, and alternative healing methods (e.g., herbal medicine, acupuncture, homeopathy) have not been adequately tested in COPD patients and thus cannot be recommended at this time.
The principal goals of pulmonary rehabilitation are to reduce symptoms, improve quality of life, and increase physical and emotional participation in everyday activities. To accomplish these goals, pulmonary rehabilitation covers a range of non-pulmonary problems that may not be adequately addressed by medical therapy for COPD. Such problems, which especially affect patients with Stage II: Moderate COPD and Stage III: Severe COPD, include exercise de-conditioning, relative social isolation, altered mood states (especially depression), muscle wasting, and weight loss. These problems have complex interrelationships and improvement in any one of these inter-linked processes can interrupt the “vicious circle” in COPD so that positive gains occur in all aspects of the illness (Figure 5-3-9).
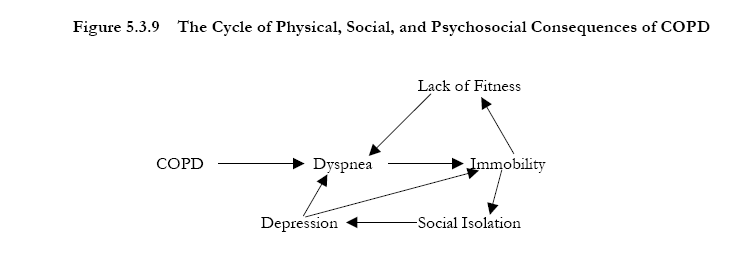
Pulmonary rehabilitation has been carefully evaluated in a large number of clinical trials; the various benefits are summarized in Figure 5-3-105,110-120.
Figure 5-3-10. Benefits of Pulmonary Rehabilitation in COPD
· Improves exercise capacity (Evidence A).
· Reduces the perceived intensity of breathlessness (Evidence A).
· Can improve health-related quality of life (Evidence A).
· Reduces the number of hospitalizations and days in the hospital (Evidence A).
· Reduces anxiety and depression associated with COPD (Evidence A).
· Strength and endurance training of the upper limbs improves arm function (Evidence B).
· Benefits extend well beyond the immediate period of training (Evidence B).
· Improves survival (Evidence B).
· Respiratory muscle training is beneficial, especially when combined with general exercise training (Evidence C).
· Psychosocial intervention is helpful (Evidence C).
Patient selection and program design. Although more information is needed on criteria for patient selection for pulmonary rehabilitation programs, COPD patients at all stages of disease appear to benefit from exercise training programs, improving with respect to both exercise tolerance and symptoms of dyspnea and fatigue121 (Evidence A). Data suggest that these benefits can be sustained even after a single pulmonary rehabilitation program122-124. Benefit does wane after a rehabilitation program ends, but if exercise training is maintained at home the patient’s health status remains above pre-rehabilitation levels (Evidence B). To date there is no consensus on whether repeated rehabilitation courses enable patients to sustain the benefits gained through the initial course.
Ideally, pulmonary rehabilitation should involve several types of health professionals. Significant benefits can also occur with more limited personnel, as long as dedicated professionals are aware of the needs of each patient. Benefits have been reported from rehabilitation programs conducted in inpatient, outpatient, and home settings114,115,125. Considerations of cost and availability most often determine the choice of setting. The educational and exercise training components of rehabilitation are usually conducted in groups, normally with 6 to 8 individuals per class (Evidence D).
The following points summarize current knowledge of considerations important in choosing patients:
· Functional status: Benefits have been seen in patients with a wide range of disability, although those who are chair-bound appear unlikely to respond even to home visiting programs126 (Evidence A).
· Severity of dyspnea: Stratification by breathlessness intensity using the MRC questionnaire (Figure 5-1-3) may be helpful in selecting patients most likely to benefit from rehabilitation. Those with MRC grade 5 dyspnea may not benefit126 (Evidence B).
· Motivation: Selecting highly motivated participants is especially important in the case of outpatient programs123.
· Smoking status: There is no evidence that smokers will benefit less than nonsmokers, but many clinicians believe that inclusion of a smoker in a rehabilitation program should be conditional on their participation in a smoking cessation program. Some data indicate that continuing smokers are less likely to complete pulmonary rehabilitation programs than nonsmokers123 (Evidence B).
Components of pulmonary rehabilitation programs. The components of pulmonary rehabilitation vary widely from program to program but a comprehensive pulmonary rehabilitation program includes exercise training, nutrition counseling, and education.
Exercise training. Exercise tolerance can be assessed by either bicycle ergometry or treadmill exercise with the measurement of a number of physiological variables, including maximum oxygen consumption, maximum heart rate, and maximum work performed. A less complex approach is to use a self-paced, timed walking test (e.g., 6-minute walking distance). These tests require at least one practice session before data can be interpreted. Shuttle walking tests offer a compromise: they provide more complete information than an entirely self-paced test, but are simpler to perform than a treadmill test127.
Exercise training ranges in frequency from daily to weekly, in duration from 10 minutes to 45 minutes per session, and in intensity from 50% peak oxygen consumption (VO2 max) to maximum tolerated128. The optimum length for an exercise program has not been investigated in randomized, controlled trials. Thus, the length depends on the resources available and usually ranges from 4 to 10 weeks, with longer programs resulting in larger effects than shorter programs113.
Participants are often encouraged to achieve a predetermined target heart rate129, but this goal may have limitations in COPD. In many programs, especially those using simple corridor exercise training, the patient is encouraged to walk to a symptom-limited maximum, rest, and then continue walking until 20 minutes of exercise have been completed. Many physicians advise patients unable to participate in a structured program to exercise on their own (e.g., walking 20 minutes daily). The benefits of this general advice have not been tested, but it is reasonable to offer such advice to patients if a formal program is not available.
Some programs also include upper limb exercises, usually involving an upper limb ergometer or resistive training with weights. There are no randomized clinical trial data to support the routine inclusion of these exercises, but they may be helpful in patients with co-morbidities that restrict other forms of exercise130,131. The addition of upper limb exercises or other strength training to aerobic training is effective in improving strength, but does not improve quality of life or exercise tolerance132.
Nutrition counseling. Nutritional state is an important determinant of symptoms, disability, and prognosis in COPD; both overweight and underweight can be a problem. Specific nutritional recommendations for patients with COPD are based on expert opinion and some small randomized clinical trials. Approximately 25% of patients with Stage II: Moderate COPD to Stage III: Severe COPD show a reduction in both their body mass index and fat free mass133-135. A reduction in body mass index is an independent risk factor for mortality in COPD patients136-138 (Evidence A).
Healthcare workers should identify and correct the reasons for reduced calorie intake in COPD patients. Patients who become breathless while eating should be advised to take small, frequent meals. Poor dentition should be corrected and co-morbidities (pulmonary sepsis, lung tumors, etc.) should be managed appropriately.
Improving the nutritional state of weight-losing COPD patients can lead to improved respiratory muscle strength139-141. However, controversy remains as to whether this additional effort is cost effective139,140. Present evidence suggests that nutritional supplementation alone may not be a sufficient strategy. Increased calorie intake is best accompanied by exercise regimes that have a nonspecific anabolic action. This approach has not been formally tested in large numbers of subjects.
Education. Most pulmonary rehabilitation programs include an educational component, but the specific contributions of education to the improvements seen after pulmonary rehabilitation remain unclear.
Assessment and follow-up
Baseline and outcome assessments of each participant in a pulmonary rehabilitation program should be made to quantify individual gains and target areas for improvement. Assessments should include:
· Detailed history and physical examination.
· Measurement of spirometry before and after a bronchodilator drug.
· Assessment of exercise capacity.
· Measurement of health status and impact of breathlessness.
· Assessment of inspiratory and expiratory muscle strength and lower limb strength (e.g., quadriceps) in patients who suffer from muscle wasting.
The first two assessments are important for establishing entry suitability and baseline status but are not used in outcome assessment. The last three assessments are baseline and outcome measures.
Several detailed questionnaires for assessing health status are available, including some that are specifically designed for patients with respiratory disease (e.g., Chronic Respiratory Disease Questionnaire48, St. George Respiratory Questionnaire142), and there is increasing evidence that these questionnaires may be useful in a clinical setting. Health status can also be assessed by generic questionnaires, such as the Medical Outcomes Study Short Form (SF36)143, to enable comparison of quality of life in different diseases.
Economic cost of rehabilitation programs. A Canadian study showing statistically significant improvements in dyspnea, fatigue, emotional health, and mastery found that the incremental cost of pulmonary rehabilitation was $11,597 (CDN) per person144. A study from the UK provided evidence that an intensive (6-week, 18-visit) multidisciplinary rehabilitation program was effective in decreasing use of health services124 (Evidence B). Although there was no difference in the number of hospital admissions between patients with disabling COPD in a control group and those who participated in the rehabilitation program, the number of days the rehabilitation group spent in the hospital was significantly lower. The rehabilitation group had more primary-care consultations at the general practitioner’s premises than did the control group, but fewer primary-care home visits. Compared with the control group, the rehabilitation group also showed greater improvements in walking ability and in general and disease-specific health status.
Oxygen therapy, one of the principal non-pharmacologic treatments for patients with Stage III: Severe COPD91,145, can be administered in three ways: long-term continuous therapy, during exercise, and to relieve acute dyspnea. The primary goal of oxygen therapy is to increase the baseline PaO2 to at least 8.0 kPa (60 mm Hg) at sea level and rest, and/or produce an SaO2 at least 90%, which will preserve vital organ function by ensuring adequate delivery of oxygen.
The long-term administration of oxygen (> 15 hours per day) to patients with chronic respiratory failure has been shown to increase survival91,146. It can also have a beneficial impact on hemodynamics, hematologic characteristics, exercise capacity, lung mechanics, and mental state147. Continuous oxygen therapy decreased resting pulmonary artery pressure in one study146 but not in another study148. Several controlled prospective studies have shown that the primary hemodynamic effect of oxygen therapy is preventing the progression of pulmonary hypertension149,150. Long-term oxygen therapy improves general alertness, motor speed, and hand grip, although the data are less clear about changes in quality of life and emotional state. The possibility of walking while using some oxygen devices may help to improve physical conditioning and have a beneficial influence on the psychological state of patients151.
Long-term oxygen therapy is generally introduced in Stage III: Severe COPD for patients who have:
· PaO2 at or below 7.3 kPa (55 mm Hg) or SaO2 at or below 88%, with or without hypercapnia (Evidence A); or
· PaO2 between 7.3 kPa (55 mm Hg) and 8.0 kPa (60 mm Hg), or SaO2 of 89%, if there is evidence of pulmonary hypertension, peripheral edema suggesting congestive cardiac failure, or polycythemia (hematocrit > 55%) (Evidence D). A decision about the use of long-term oxygen should be based on the waking PaO2 values. The prescription should always include the source of supplemental oxygen (gas or liquid), method of delivery, duration of use, and flow rate at rest, during exercise, and during sleep.
Oxygen therapy given during exercise increases walking distance and endurance, optimizing oxygen delivery to tissues and utilization by muscles. However, there are no data to suggest that long-term oxygen therapy changes exercise capacity per se. Where available, this treatment is usually restricted to patients who meet the criteria for continuous oxygen therapy, or experience significant oxygen desaturation during exercise (Evidence C).
Oxygen therapy reduces the oxygen cost of breathing and minute ventilation, a mechanism that although still disputed helps to minimize the sensation of dyspnea. This has led to the use of short burst therapy to control severe dyspnea such as occurs after climbing stairs. Often the patient keeps a cylinder of oxygen at home to use as needed. Whether this is of physiological, psychological, or any benefit at all is not known (Evidence C).
Cost considerations. Supplemental home oxygen is usually the most costly component of outpatient therapy for adults with COPD who require this therapy152. Studies of the cost effectiveness of alternative outpatient oxygen delivery methods in the US and Europe suggest that oxygen concentrator devices may be more cost effective than cylinder delivery systems153,154.
Oxygen use in air travel. Although air travel is safe for most patients with chronic respiratory failure who are on long-term oxygen therapy, patients should be instructed to increase the flow by 1-2 L/min during the flight155. Ideally, patients who fly should be able to maintain an in-flight PaO2 of at least 6.7 kPa (50 mm Hg). Studies indicate that this can be achieved in those with moderate to severe hypoxemia at sea level by supplementary oxygen at 3 L/min by nasal cannulae or 31% by Venturi face mask156. Those with a resting PaO2 at sea level of > 9.3 kPa (70 mm Hg) are likely to be safe to fly without supplementary oxygen155,157, although it is important to emphasize that a resting PaO2 > 9.3 kPa (70 mm Hg) at sea level does not exclude the development of severe hypoxemia when traveling by air (Evidence C). Careful consideration should be given to any comorbidity that may impair oxygen delivery to tissues (e.g., cardiac impairment, anemia). Also, walking along the aisle may profoundly aggravate hypoxemia158.
Although both noninvasive ventilation (using either negative or positive pressure devices) and invasive (conventional) mechanical ventilation are essentially designed to manage and treat acute episodes of COPD, for years noninvasive ventilation has been applied in patients with Stage III: Severe COPD and chronic respiratory failure. This has followed the successful use of noninvasive ventilation in other forms of chronic respiratory failure due to chest wall deformities and/or neuromuscular disorders. Several scientific studies have examined the use of ventilatory support and there is no convincing evidence that this therapy has a role in the management of stable COPD. It is possible that some patients with chronic hypercapnia may benefit from this form of treatment, but no randomized controlled study has yet been reported.
Noninvasive mechanical ventilation. This modality of ventilatory support is applied when endotracheal and nasotracheal ventilation are not needed, using either negative pressure ventilation (nPV) or noninvasive intermittent positive pressure ventilation (NIPPV).
Noninvasive negative pressure ventilation (nPV). The use of tank respirators, cuirass, or poncho ventilation is largely of historical interest in COPD. Problems with patient comfort and limited access restrict future use of nPV159,160. When this treatment is used in chronic respiratory failure, some patients develop upper airway obstruction during sleep161. A comparison of domiciliary active and sham nPV in patients with chronic respiratory failure due to COPD showed no differences in shortness of breath, exercise tolerance, arterial blood gases, respiratory muscle strength, or quality of life between the two treatments162.
Noninvasive intermittent positive pressure ventilation (NIPPV). The role of NIPPV in chronic respiratory failure remains unsettled, although this is now the standard means of providing noninvasive ventilatory support in other instances of chronic respiratory failure not directly related to COPD. NIPPV can be delivered by different types of ventilators: volume-controlled, pressure-controlled, bilevel positive airway pressure, or continuous positive airway pressure. New devices with lower cost, greater ease of operation, and greater portability are constantly being developed163. Recent technical improvements have facilitated the use of NIPPV while reducing the possibility of air leaking through face or nasal masks.
A study of NIPPV compared to conventional therapy in a population with end-stage COPD using a randomized, crossover design for a 3-month period found that the noninvasive approach is not well tolerated and is associated with marginal clinical and functional improvements164 (Evidence B). The use of NIPPV together with long-term oxygen therapy in a randomized crossover study in a small subset of patients produced a significant improvement in daytime arterial blood gases, total sleep time, sleep efficiency, quality of life, and overnight PaCO2 compared with oxygen therapy alone, indicating that NIPPV may be a useful addition to long-term oxygen therapy165 (Evidence B). However, a similar approach in a larger series of patients concluded that NIPPV plus long-term oxygen therapy does not improve long-term survival. In this study, however, intensive care admissions were reduced and exercise capacity was improved166 (Evidence C).
Given this conflicting evidence, long-term NIPPV cannot be recommended for the routine treatment of patients with chronic respiratory failure due to COPD. Nonetheless, the combination of NIPPV with long-term oxygen therapy may be of some use in a selected subset of patients, particularly in those with pronounced daytime hypercapnia167.
Invasive (conventional) mechanical ventilation. The appropriateness of using invasive (conventional) ventilation in end-stage COPD continues to be debated. There are no guidelines to define which patients will benefit.
Bullectomy. Bullectomy is an older surgical procedure for bullous emphysema. By removing a large bulla that does not contribute to gas exchange, the adjacent lung parenchyma is decompressed. Bullectomy can be performed thoracoscopically. In carefully selected patients, this procedure is effective in reducing dyspnea and improving lung function168 (Evidence C).
Bullae may be removed to alleviate local symptoms such as hemoptysis, infection, or chest pain, and to allow re-expansion of a compressed lung region. This is the usual indication in patients with COPD. In considering the possible benefit of surgery it is crucial to estimate the effect of the bulla on the lung and the function of the non-bullous lung. A thoracic CT scan, arterial blood gas measurement, and comprehensive respiratory function tests are essential before making a decision regarding suitability for resection of a bulla. Normal or minimally reduced diffusing capacity, absence of significant hypoxemia, and evidence of regional reduction in perfusion with good perfusion in the remaining lung are indications a patient will likely benefit from surgery169. However, pulmonary hypertension, hypercapnia, and severe emphysema are not absolute contraindications for bullectomy. Some investigators have recommended that the bulla must occupy 50% or more of the hemithorax and produce definite displacement of the adjacent lung before surgery is performed170.
Lung volume reduction surgery (LVRS). LVRS is a surgical procedure in which parts of the lung are resected to reduce hyperinflation171, making respiratory muscles more effective pressure generators by improving their mechanical efficiency (as measured by length/tension relationship, curvature of the diaphragm, and area of apposition)172,173. In addition, LVRS increases the elastic recoil pressure of the lung and thus improves expiratory flow rates174.
In some centers with adequate experience, perioperative mortality of LVRS has been reported to be less than 5%. Results have been reported following bilateral (upper parts) resection using median sternotomy175,176 or video-assisted thoracoscopy (VATS)177. Most studies select patients with FEV1 < 35% predicted, PaCO2 < 6.0 kPa (45 mm Hg), predominant upper lobe emphysema on CT scan, and a residual volume of > 200% predicted. The average increase in FEV1 following LVRS has ranged from 32% to 93%, and the decrease in TLC from 15% to 20%175,178. LVRS appears to improve exercise capacity as well as quality of life in some patients. There are reports of these effects lasting more than one year175-177.
Hospital costs associated with LVRS in 52 consecutive patients179 ranged from $11,712 to $121,829 (US). Hospital charges in 23 consecutive patients admitted for LVRS at a single institution180 ranged from $20,032 to $75,561 with a median charge of $26,669 (US). A small number of individuals incurred extraordinary costs because of complications. Advanced age was a significant factor leading to higher expected total hospital costs.
Although there are some encouraging reports181, LVRS is still an experimental palliative surgical procedure182. Most results (Evidence C) reported to date are from uncontrolled studies; several large randomized multicenter studies are underway to investigate the effectiveness and cost of LVRS in comparison to vigorous conventional therapy183. Until the results of these controlled studies are known, LVRS can- not be recommended for widespread use.
Lung transplantation. In appropriately selected patients with very advanced COPD, lung transplantation has been shown to improve quality of life and functional capacity184-187(Evidence C), although the Joint United Network for Organ Sharing in 1998 found that lung transplantation does not confer a survival benefit in patients with end-stage emphysema after two years186. Criteria for referral for lung transplantation include FEV1 < 35% predicted, PaO2 < 7.3-8.0 kPa (55-60 mm Hg), PaCO2 > 6.7 kPa (50 mm Hg), and secondary pulmonary hypertension188,189.
Lung transplantation is limited by the shortage of donor organs, which has led some centers to adopt the single lung technique. The common complications seen in COPD patients after lung transplantation, apart from operative mortality, are acute rejection and bronchiolitis obliterans, CMV, other opportunistic fungal (Candida, Aspergillus, Cryptococcus, Carini) or bacterial (Pseudomonas, Staphylococcus species) infections, lymphoproliferative disease, and lymphomas185.
Another limitation of lung transplantation is its cost. Hospitalization costs associated with lung transplantation have ranged from $110,000 to well over $200,000 (US). Costs remain elevated for months to years after surgery due to the high cost of complications and the immunosuppressive regimens190-193 that must be initiated during or immediately after surgery.
1. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995; 122:823-32.
2. Janelli LM, Scherer YK, Schmieder LE. Can a pulmonary health teaching program alter patients’ ability to cope with COPD? Rehabil Nurs 1991; 16:199-202.
3. Ashikaga T, Vacek PM, Lewis SO. Evaluation of a community-based education program for individuals with chronic obstructive pulmonary disease. J Rehabil 1980; 46:23-7.
4. Toshima MT, Kaplan RM, Ries AL. Experimental evaluation of rehabilitation in chronic obstructive pulmonary disease: short-term effects on exercise endurance and health status. Health Psychol 1990; 9:237-52.
5. Celli BR. Pulmonary rehabilitation in patients with COPD. Am J Respir Crit Care Med 1995; 152:861-4.
6. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994; 272:1497-505.
7. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995; 152:1423-33.
8. Clark NM, Nothwehr F, Gong M, Evans D, Maiman LA, Hurwitz ME, et al. Physician-patient partnership in managing chronic illness. Acad Med 1995; 70:957-9.
9. Heffner JE, Fahy B, Hilling L, Barbieri C. Outcomes of advance directive education of pulmonary rehabilitation patients. Am J Respir Crit Care Med 1997; 155:1055-9.
10. Tougaard L, Krone T, Sorknaes A, Ellegaard H. Economic benefits of teaching patients with chronic obstructive pulmonary disease about their illness. The PASTMA Group. Lancet 1992; 339:1517-20.
11. Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999; 340:1948-53.
12. Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999; 353:1819-23.
13. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320:1297-303.
14. Calverley PMA. Symptomatic bronchodilator treatment. In: Calverley PMA, Pride NB, eds. Chronic obstructive pulmonary disease. London: Chapman and Hall; 1995. p. 419-45.
15. Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:967-75.
16. Berger R, Smith D. Effect of inhaled metaproterenol on exercise performance in patients with stable “fixed” airway obstruction. Am Rev Respir Dis 1988; 138:624-9.
17. Hay JG, Stone P, Carter J, Church S, Eyre-Brook A, Pearson MG, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J 1992; 5:659-64.
18. Vathenen AS, Britton JR, Ebden P, Cookson JB, Wharrad HJ, Tattersfield AE. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis 1988; 138:850-5.
19. Gross NJ, Petty TL, Friedman M, Skorodin MS, Silvers GW, Donohue JF. Dose response to ipratropium as a nebulized solution in patients with chronic obstructive pulmonary disease. A three-center study. Am Rev Respir Dis 1989; 139:1188-91.
20. Chrystyn H, Mulley BA, Peake MD. Dose response relation to oral theophylline in severe chronic obstructive airways disease. BMJ 1988; 297:1506-10.
21. Higgins BG, Powell RM, Cooper S, Tattersfield AE. Effect of salbutamol and ipratropium bromide on airway calibre and bronchial reactivity in asthma and chronic bronchitis. Eur Respir J 1991; 4:415-20.
22. Ericsson CH, Svartengren K, Svartengren M, Mossberg B, Philipson K, Blomquist M, et al. Repeatability of airway deposition and tracheobronchial clearance rate over three days in chronic bronchitis. Eur Respir J 1995; 8:1886-93.
23. Kim CS, Kang TC. Comparative measurement of lung deposition of inhaled fine particles in normal subjects and patients with obstructive airway disease. Am J Respir Crit Care Med 1997; 155:899-905.
24. O’Driscoll BR, Kay EA, Taylor RJ, Weatherby H, Chetty MC, Bernstein A. A long-term prospective assessment of home nebulizer treatment. Respir Med 1992; 86:317-25.
25. Jenkins SC, Heaton RW, Fulton TJ, Moxham J. Comparison of domiciliary nebulized salbutamol and salbutamol from a metered-dose inhaler in stable chronic airflow limitation. Chest 1987; 91:804-7.
26. COMBIVENT Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. Chest 1994; 105:1411-9.
27. van Schayck CP, Folgering H, Harbers H, Maas KL, van Weel C. Effects of allergy and age on responses to salbutamol and ipratropium bromide in moderate asthma and chronic bronchitis.Thorax 1991; 46:355-9.
28. Ulrik CS. Efficacy of inhaled salmeterol in the management of smokers with chronic obstructive pulmonary disease: a single centre randomised, double blind, placebo controlled, crossover study.Thorax 1995; 50:750-4.
29. Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) [published erratum appears in Eur Respir J 1997; 10:1696]. Eur Respir J 1997; 10:815-21.
30. Cazzola M, Matera MG, Santangelo G, Vinciguerra A, Rossi F, D’Amato G. Salmeterol and formoterol in partially reversible severe chronic obstructive pulmonary disease: a dose-response study. Respir Med 1995; 89:357-62.
31. Ikeda A, Nishimura K, Koyama H, Izumi T. Bronchodilating effects of combined therapy with clinical dosages of ipratropium bromide and salbutamol for stable COPD: comparison with ipratropium bromide alone. Chest 1995; 107:401-5.
32. Guyatt GH, Townsend M, Pugsley SO, Keller JL, Short HD, Taylor DW, et al. Bronchodilators in chronic air-flow limitation. Effects on airway function, exercise capacity, and quality of life. Am Rev Respir Dis 1987; 135:1069-74.
33. Jones PW, Bosh TK. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med 1997; 155:1283-9.
34. Mahler DA, Donohue JF, Barbee RA, Goldman MD, Gross NJ, Wisniewski ME, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115:957-65.
35. Shim CS, Williams MH Jr. Bronchodilator response to oral aminophylline and terbutaline versus aerosol albuterol in patients with chronic obstructive pulmonary disease. Am J Med 1983; 75:697-701.
36. Lipworth BJ, McDevitt DG, Struthers AD. Hypokalemic and ECG sequelae of combined beta-agonist/diuretic therapy. Protection by conventional doses of spironolactone but not triamterene. Chest 1990; 98:811-5.
37. Uren NG, Davies SW, Jordan SL, Lipkin DP. Inhaled bronchodilators increase maximum oxygen consumption in chronic left ventricular failure. Eur Heart J 1993; 14:744-50.
38. Khoukaz G, Gross NJ. Effects of salmeterol on arterial blood gases in patients with stable chronic obstructive pulmonary disease. Comparison with albuterol and ipratropium. Am J Respir Crit Care Med 1999; 160:1028-30.
39. Barnes PJ. Bronchodilators: basic pharmacology. In: Calverley PMA, Pride NB, eds. Chronic obstructive pulmonary disease. London: Chapman and Hall; 1995. p. 391-417.
40. Aubier M. Pharmacotherapy of respiratory muscles. Clin Chest Med 1988; 9:311-24.
41. Moxham J. Aminophylline and the respiratory muscles: an alternative view. Clin Chest Med 1988; 9:325-36.
42. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med 1989; 320:1521-5.
43. McKay SE, Howie CA, Thomson AH, Whiting B, Addis GJ. Value of theophylline treatment in patients handicapped by chronic obstructive lung disease.Thorax 1993; 48:227-32.
44. Taylor DR, Buick B, Kinney C, Lowry RC, McDevitt DG. The efficacy of orally administered theophylline, inhaled salbutamol, and a combination of the two as chronic therapy in the management of chronic bronchitis with reversible air-flow obstruction. Am Rev Respir Dis 1985; 131:747-51.
45. The COMBIVENT Inhalation Solution Study Group. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 1997; 112:1514-21.
46. Gross N, Tashkin D, Miller R, Oren J, Coleman W, Linberg S. Inhalation by nebulization of albuterol-ipratropium combination (Dey combination) is superior to either agent alone in the treatment of chronic obstructive pulmonary disease. Dey Combination Solution Study Group. Respiration 1998; 65:354-62.
47. van Noord JA, de Munck DR, Bantje TA, Hop WC, Akveld ML, Bommer AM. Long-term treatment of chronic obstructive pulmonary disease with salmeterol and the additive effect of ipratropium. Eur Respir J 2000; 15:878-85.
48. Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease.Thorax 1987; 42:773-8.
49. Easton PA, Jadue C, Dhingra S, Anthonisen NR. A comparison of the bronchodilating effects of a beta-2 adrenergic agent (albuterol) and an anticholinergic agent (ipratropium bromide), given by aerosol alone or in sequence. N Engl J Med 1986; 315:735-9.
50. Friedman M, Serby CW, Menjoge SS, Wilson JD, Hilleman DE, Witek TJ Jr. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999; 115:635-41.
51. Tashkin DP, Bleecker E, Braun S, Campbell S, DeGraff AC Jr, Hudgel DW, et al. Results of a multicenter study of nebulized inhalant bronchodilator solutions. Am J Med 1996; 100:62S-9.
52. Callahan CM, Dittus RS, Katz BP. Oral corticosteroid therapy for patients with stable chronic obstructive pulmonary disease. A meta-analysis. Ann Intern Med 1991; 114:216-23.
53. Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. The Danish Society of Respiratory Medicine. Respir Med 1999; 93:715-8.
54. Postma DS, Peters I, Steenhuis EJ, Sluiter HJ. Moderately severe chronic airflow obstruction. Can corticosteroids slow down obstruction? Eur Respir J 1988; 1:22-6.
55. Postma DS, Steenhuis EJ, van der Weele LT, Sluiter HJ. Severe chronic airflow obstruction: can corticosteroids slow down progression? Eur J Respir Dis 1985; 67:56-64.
56. Decramer M, de Bock V, Dom R. Functional and histologic picture of steroid-induced myopathy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:1958-64.
57. Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med 1994; 150:11-6.
58. Decramer M, Stas KJ. Corticosteroid-induced myopathy involving respiratory muscles in patients with chronic obstructive pulmonary disease or asthma. Am Rev Respir Dis 1992; 146:800-2.
59. Confalonieri M, Mainardi E, Della Porta R, Bernorio S, Gandola L, Beghe B, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease.Thorax 1998; 53:583-5.
60. Wilcke JT, Dirksen A. The effect of inhaled glucocortico- steroids in emphysema due to alpha1-antitrypsin deficiency. Respir Med 1997; 91:275-9.
61. Rutgers SR, Koeter GH, van der Mark TW, Postma DS. Short-term treatment with budesonide does not improve hyperresponsiveness to adenosine 5’-monophosphate in COPD. Am J Respir Crit Care Med 1998; 157:880-6.
62. Weiner P, Weiner M, Azgad Y, Zamir D. Inhaled budesonide therapy for patients with stable COPD. Chest 1995; 108:1568-71.
63. Chan CH, Cohen M, Pang J. The effects of inhaled corticosteroids on chronic airflow limitation. Asian Pac J Allergy Immunol 1993; 11:97-101.
64. Wesseling GJ, Quaedvlieg M, Wouters EF. Inhaled budesonide in chronic bronchitis. Effects on respiratory impedance. Eur Respir J 1991; 4:1101-5.
65. Engel T, Heinig JH, Madsen O, Hansen M, Weeke ER. A trial of inhaled budesonide on airway responsiveness in smokers with chronic bronchitis. Eur Respir J 1989; 2:935-9.
66. Boothman-Burrell D, Delany SG, Flannery EM, Hancox RJ, Taylor DR. The efficacy of inhaled corticosteroids in the management of non asthmatic chronic airflow obstruction. N Z Med J 1997; 110:370-3.
67. Shim CS, Williams MH Jr. Aerosol beclomethasone in patients with steroid-responsive chronic obstructive pulmonary disease. Am J Med 1985; 78:655-8.
68. Nishimura K, Koyama H, Ikeda A, Tsukino M, Hajiro T, Mishima M, et al. The effect of high-dose inhaled beclomethasone dipropionate in patients with stable COPD. Chest 1999; 115:31-7.
69. Weir DC, Gove RI, Robertson AS, Burge PS. Response to corticosteroids in chronic airflow obstruction: relationship to emphysema and airways collapse. Eur Respir J 1991; 4:1185-90.
70. Watson A, Lim TK, Joyce H, Pride NB. Failure of inhaled corticosteroids to modify bronchoconstrictor or bronchodilator responsiveness in middle-aged smokers with mild airflow obstruction. Chest 1992; 101:350-5.
71. Auffarth B, Postma DS, de Monchy JG, van der Mark TW, Boorsma M, Koeter GH. Effects of inhaled budesonide on spirometric values, reversibility, airway responsiveness, and cough threshold in smokers with chronic obstructive lung disease.Thorax 1991; 46:372-7.
72. Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group [published erratum appears in Lancet 1998; 351:1968]. Lancet 1998; 351:773-80.
73. The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000; 343:1902-9.
74. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, National Institutes of Health; 1997. Available from: URL: http://www.nhlbi.nih.gov
75. Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med 1994; 331:778-84.
76. Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis 1994; 169:68-76.
77. Hak E, van Essen GA, Buskens E, Stalman W, de Melker RA. Is immunising all patients with chronic lung disease in the community against influenza cost effective? Evidence from a general practice based clinical prospective cohort study in Utrecht, the Netherlands. J Epidemiol Community Health 1998; 52:120-5.
78. Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986; 315:1318-27.
79. Williams JH, Jr., Moser KM. Pneumococcal vaccine and patients with chronic lung disease. Ann Intern Med 1986; 104:106-9.
80. Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest 1987; 92:204-12.
81. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis: influence of penicillin and tetracylcline administered daily, or intermittently for exacerbations. BMJ 1961; 2:979-85.
82. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: influence of daily penicillin and tetracycline on exacerbations and their cost. A report to the research committee of the British Tuberculosis Assoication by their Chronic Bronchitis Subcommittee. BMJ 1960; 1:297-303.
83. Fletcher CM, Ball JD, Carstairs LW, Couch AHC, Crofton JM, Edge JR, et al. Value of chemoprophylaxis and chemotherapy in early chronic bronchitis. A report to the Medical Research Council by their Working Party on trials of chemotherapy in early chronic bronchitis. BMJ 1966; 1:1317-22.
84. Johnston RN, McNeill RS, Smith DH, Dempster MB, Nairn JR, Purvis MS, et al. Five-year winter chemoprophylaxis for chronic bronchitis. BMJ 1969; 4:265-9.
85. Isada CM, Stoller JK. Chronic bronchitis: the role of antibiotics. In: Niederman MS, Sarosi GA, Glassroth J, eds. Respiratory infections: a scientific basis for management. London: WB Saunders; 1994. p. 621-33.
86. Siafakas NM, Bouros D. Management of acute exacerbation of chronic obstructive pulmonary disease. In: Postma DS, Siafakas NM, eds. Management of chronic obstructive pulmonary disease. Sheffield: ERS Monograph; 1998. p. 264-77.
87. Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double-blind, placebo-controlled trial. Respiration 1996; 63:174-80.
88. Guyatt GH, Townsend M, Kazim F, Newhouse MT. A controlled trial of ambroxol in chronic bronchitis. Chest 1987; 92:618-20.
89. Petty TL. The National Mucolytic Study. Results of a randomized, double-blind, placebo-controlled study of iodinated glycerol in chronic obstructive bronchitis. Chest 1990; 97:75-83.
90. Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2000; 2: Available from: URL: www.update-software.com or www.updatusa.com
91. Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 1995; 8:1398-420.
92. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis 1987; 136:225-44.
93. Hansen NC, Skriver A, Brorsen-Riis L, Balslov S, Evald T, Maltbaek N, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994; 88:531-5.
94. British Thoracic Society Research Committee. Oral N-acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airways obstruction.Thorax 1985; 40:832-5.
95. Boman G, Backer U, Larsson S, Melander B, Wahlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983; 64:405-15.
96. Rasmussen JB, Glennow C. Reduction in days of illness after long-term treatment with N- acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J 1988; 1:351-5.
97. Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. The PARI-IS Study Steering Committee and Research Group. Prevention of acute respiratory infection by an immunostimulant . Am J Respir Crit Care Med 1997; 156:1719-24.
98. Anthonisen NR. OM-8BV for COPD [editorial; comment]. Am J Respir Crit Care Med 1997; 156:1713-4.
99. Irwin RS, Boulet LP, Cloutier MM, Fuller R, Gold PM, Hoffstein V, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998; 114:133-81S.
100. Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 1996; 347:436-40.
101. Jones AT, Evans TW. NO: COPD and beyond.Thorax 1997; 52 Suppl 3:S16-21.
102. Watanabe S, Kanner RE, Cutillo AG, Menlove RL, Bachand RT Jr, Szalkowski MB, et al. Long-term effect of almitrine bismesylate in patients with hypoxemic chronic obstructive pulmonary disease. Am Rev Respir Dis 1989; 140:1269-73.
103. Bardsley PA, Howard P, DeBacker W, Vermeire P, Mairesse M, Ledent C, et al. Two years treatment with almitrine bismesylate in patients with hypoxic chronic obstructive airways disease. Eur Respir J 1991; 4:308-10.
104. Winkelmann BR, Kullmer TH, Kneissl DG, Trenk D, Kronenberger H. Low-dose almitrine bismesylate in the treatment of hypoxemia due to chronic obstructive pulmonary disease. Chest 1994; 105:1383-91.
105. Eiser N, Denman WT, West C, Luce P. Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the “pink puffer” syndrome. Eur Respir J 1991; 4:926-31.
106. Young IH, Daviskas E, Keena VA. Effect of low dose nebulised morphine on exercise endurance in patients with chronic lung disease.Thorax 1989; 44:387-90.
107. Woodcock AA, Gross ER, Gellert A, Shah S, Johnson M, Geddes DM. Effects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gases. N Engl J Med 1981; 305:1611-6.
108. Rice KL, Kronenberg RS, Hedemark LL, Niewoehner DE. Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstruction. Br J Dis Chest 1987; 81:287-92.
109. Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1877-80.
110. American Thoracic Society. Pulmonary rehabilitation-1999. Am J Respir Crit Care Med 1999; 159:1666-82.
111. Fishman AP. Pulmonary rehabilitation research. Am J Respir Crit Care Med 1994; 149:825-33.
112. ACCP/AACVPR Pulmonary Rehabilitation Guidelines Panel, American College of Chest Physicians and American Association of Cardiovascular and Pulmonary Rehabilitation. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guidelines. Chest 1997; 112:1363-96.
113. Lacasse Y, Wong E, Guyatt GH, King D, Cook DJ, Goldstein RS. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996; 348:1115-9.
114. Goldstein RS, Gort EH, Stubbing D, Avendano MA, Guyatt GH. Randomised controlled trial of respiratory rehabilitation. Lancet 1994; 344:1394-7.
115. Wijkstra PJ, Van Altena R, Kraan J, Otten V, Postma DS, Koeter GH. Quality of life in patients with chronic obstructive pulmonary disease improves after rehabilitation at home. Eur Respir J 1994; 7:269-73.
116. O’Donnell DE, McGuire M, Samis L, Webb KA. The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Respir Crit Care Med 1995; 152:2005-13.
117. Lake FR, Henderson K, Briffa T, Openshaw J, Musk AW. Upper-limb and lower-limb exercise training in patients with chronic airflow obstruction. Chest 1990; 97:1077-82.
118. Ries AL, Ellis B, Hawkins RW. Upper extremity exercise training in chronic obstructive pulmonary disease. Chest 1988; 93:688-92.
119. Martinez FJ, Vogel PD, Dupont DN, Stanopoulos I, Gray A, Beamis JF. Supported arm exercise vs. unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993; 103:1397-402.
120. Wijkstra PJ, Ten Vergert EM, van Altena R, Otten V, Kraan J, Postma DS, et al. Long term benefits of rehabilitation at home on quality of life and exercise tolerance in patients with chronic obstructive pulmonary disease.Thorax 1995; 50:824-8.
121. Berry MJ, Rejeski WJ, Adair NE, Zaccaro D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. Am J Respir Crit Care Med 1999; 160:1248-53.
122. Foglio K, Bianchi L, Bruletti G, Battista L, Pagani M, Ambrosino N. Long-term effectiveness of pulmonary rehabilitation in patients with chronic airway obstruction. Eur Respir J 1999; 13:125-32.
123. Young P, Dewse M, Fergusson W, Kolbe J. Improvements in outcomes for chronic obstructive pulmonary disease (COPD) attributable to a hospital-based respiratory rehabilitation programme. Aust N Z J Med 1999; 29:59-65.
124. Griffiths TL, Burr ML, Campbell IA, Lewis-Jenkins V, Mullins J, Shiels K, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial [published erratum appears in Lancet 2000; 355:1280]. Lancet 2000; 355:362-8.
125. McGavin CR, Gupta SP, Lloyd EL, McHardy GJ. Physical rehabilitation for the chronic bronchitic: results of a controlled trial of exercises in the home.Thorax 1977; 32:307-11.
126. Wedzicha JA, Bestall JC, Garrod R, Garnham R, Paul EA, Jones PW. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998; 12:363-9.
127. Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction.Thorax 1992; 47:1019-24.
128. Mahler DA. Pulmonary rehabilitation. Chest 1998; 113:263-8S.
129. American College of Sports Medicine position stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Med Sci Sports Exerc 1990; 22:265-74.
130. Smith K, Cook D, Guyatt GH, Madhavan J, Oxman AD. Respiratory muscle training in chronic airflow limitation: a meta-analysis. Am Rev Respir Dis 1992; 145:533-9.
131. Belman MJ, Botnick WC, Nathan SD, Chon KH. Ventilatory load characteristics during ventilatory muscle training. Am J Respir Crit Care Med 1994; 149:925-9.
132. Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:896-901.
133. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147:1151-6.
134. Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 1994; 7:1793-7.
135. Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis 1989; 139:1435-8.
136. Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1791-7.
137. Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153:961-6.
138. Gorecka D, Gorzelak K, Sliwinski P, Tobiasz M, Zielinski J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia.Thorax 1997; 52:674-9.
139. Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1988; 137:1075-82.
140. Rogers RM, Donahoe M, Costantino J. Physiologic effects of oral supplemental feeding in malnourished patients with chronic obstructive pulmonary disease. A randomized control study. Am Rev Respir Dis 1992; 146:1511-7.
141. Whittaker JS, Ryan CF, Buckley PA, Road JD. The effects of refeeding on peripheral and respiratory muscle function in malnourished chronic obstructive pulmonary disease patients. Am Rev Respir Dis 1990; 142:283-8.
142. Jones PW, Quirk FH, Baveystock CM. The St. George’s Respiratory Questionnaire. Respir Med 1991; 85 Suppl B:25-31, discussion 3-7.
143. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473-83.
144. Goldstein RS, Gort EH, Guyatt GH, Feeny D. Economic analysis of respiratory rehabilitation. Chest 1997; 112:370-9.
145. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152:S77-121.
146. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93:391-8.
147. Tarpy SP, Celli BR. Long-term oxygen therapy. N Engl J Med 1995; 333:710-4.
148. Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1:681-6.
149. Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Pelletier A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131:493-8.
150. Zielinski J, Tobiasz M, Hawrylkiewicz I, Sliwinski P, Palasiewicz G. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients: a 6-year prospective study. Chest 1998; 113:65-70.
151. Petty TL. Supportive therapy in COPD. Chest 1998; 113:256-62S.
152. Petty TL, O’Donohue WJ Jr. Further recommendations for prescribing, reimbursement, technology development, and research in long-term oxygen therapy. Summary of the Fourth Oxygen Consensus Conference, Washington, DC, October 15-16, 1993. Am J Respir Crit Care Med 1994; 150:875-7.
153. Pelletier-Fleury N, Lanoe JL, Fleury B, Fardeau M. The cost of treating COPD patients with long-term oxygen therapy in a French population. Chest 1996; 110:411-6.
154. Heaney LG, McAllister D, MacMahon J. Cost minimisation analysis of provision of oxygen at home: are the drug tariff guidelines cost effective? BMJ 1999; 319:19-23.
155. Gong H Jr. Air travel and oxygen therapy in cardiopulmonary patients. Chest 1992; 101:1104-13.
156. Berg BW, Dillard TA, Rajagopal KR, Mehm WJ. Oxygen supplementation during air travel in patients with chronic obstructive lung disease. Chest 1992; 101:638-41.
157. Gong H Jr, Tashkin DP, Lee EY, Simmons MS. Hypoxia-altitude simulation test. Evaluation of patients with chronic airway obstruction. Am Rev Respir Dis 1984; 130:980-6.
158. Christensen CC, Ryg M, Refvem OK, Skjonsberg OH. Development of severe hypoxaemia in chronic obstructive pulmonary disease patients at 2,438 m (8,000 ft) altitude. Eur Respir J 2000; 15:635-9.
159. Muir JF. Pulmonary rehabilitation in chronic respiratory insufficiency. 5. Home mechanical ventilation.Thorax 1993; 48:1264-73.
160. Simonds AK. Negative pressure ventilation in acute hypercapnic chronic obstructive pulmonary disease [editorial; comment].Thorax 1996; 51:1069-70.
161. Corrado A, Gorini M, Villella G, De Paola E. Negative pressure ventilation in the treatment of acute respiratory failure: an old noninvasive technique reconsidered. Eur Respir J 1996; 9:1531-44.
162. Shapiro SH, Ernst P, Gray-Donald K, Martin JG, Wood-Dauphinee S, Beaupre A, et al. Effect of negative pressure ventilation in severe chronic obstructive pulmonary disease. Lancet 1992; 340:1425-9.
163. Hillberg RE, Johnson DC. Noninvasive ventilation. N Engl J Med 1997; 337:1746-52.
164. Strumpf DA, Millman RP, Carlisle CC, Grattan LM, Ryan SM, Erickson AD, et al. Nocturnal positive-pressure ventilation via nasal mask in patients with severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 144:1234-9.
165. Meecham Jones DJ, Paul EA, Jones PW, Wedzicha JA. Nasal pressure support ventilation plus oxygen compared with oxygen therapy alone in hypercapnic COPD. Am J Respir Crit Care Med 1995; 152:538-44.
166. Clini E, Sturani C, Porta R, Scarduelli C, Galavotti V, Vitacca M, et al. Outcome of COPD patients performing nocturnal non-invasive mechanical ventilation. Respir Med 1998; 92:1215-22.
167. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation - a consensus conference report. Chest 1999; 116:521-34.
168. Mehran RJ, Deslauriers J. Indications for surgery and patient work-up for bullectomy. Chest Surg Clin N Am 1995; 5:717-34.
169. Hughes JA, MacArthur AM, Hutchison DC, Hugh-Jones P. Long term changes in lung function after surgical treatment of bullous emphysema in smokers and ex-smokers.Thorax 1984; 39:140-2.
170. Laros CD, Gelissen HJ, Bergstein PG, Van den Bosch JM, Vanderschueren RG, Westermann CJ, et al. Bullectomy for giant bullae in emphysema. J Thorac Cardiovasc Surg 1986; 91:63-70.
171. Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, et al. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995; 109:106-16,; discussion 16-9.
172. Criner G, Cordova FC, Leyenson V, Roy B, Travaline J, Sudarshan S, et al. Effect of lung volume reduction surgery on diaphragm strength. Am J Respir Crit Care Med 1998; 157:1578-85.
173. Martinez FJ, de Oca MM, Whyte RI, Stetz J, Gay SE, Celli BR. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med 1997; 155:1984-90.
174. Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998; 157:715-22.
175. Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996; 112:1319-29.
176. Russi EW, Stammberger U, Weder W. Lung volume reduction surgery for emphysema. Eur Respir J 1997; 10:208-18.
177. Gelb AF, McKenna RJ Jr, Brenner M, Schein MJ, Zamel N, Fischel R. Lung function 4 years after lung volume reduction surgery for emphysema. Chest 1999; 116:1608-15.
178. Brenner M, McKenna RJ Jr, Gelb AF, Fischel RJ, Wilson AF. Rate of FEV1 change following lung volume reduction surgery. Chest 1998; 113:652-9.
179. Elpern EH, Behner KG, Klontz B, Warren WH, Szidon JP, Kesten S. Lung volume reduction surgery: an analysis of hospital costs. Chest 1998; 113:896-9.
180. Albert RK, Lewis S, Wood D, Benditt JO. Economic aspects of lung volume reduction surgery. Chest 1996; 110:1068-71.
181. Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med 2000; 343:239-45.
182. Benditt JO, Albert RK. Surgical options for patients with advanced emphysema. Clin Chest Med 1997; 18:577-93.
183. Rationale and design of the National Emphysema Treatment Trial (NETT): A prospective randomized trial of lung volume reduction surgery. J Thorac Cardiovasc Surg 1999; 118:518-28.
184. Trulock EP. Lung transplantation. Am J Respir Crit Care Med 1997; 155:789-818.
185. Theodore J, Lewiston N. Lung transplantation comes of age [editorial; comment]. N Engl J Med 1990; 322:772-4.
186. Hosenpud JD, Bennett LE, Keck BM, Fiol B, Boucek MM, Novick RJ. The registry of the International Society for Heart and Lung Transplantation: fifteenth official report - 1998. J Heart Lung Transplant 1998; 17:656-68.
187. Annual report of the US scientific registry for transplant recipients and the Organ Procurement and Transplantation Network. Transplant data: 1988-1994. Washington, DC: Division of Transplantation, Health Resources and Services Administration, US Department of Health and Human Services; 1995.
188. Hosenpud JD, Bennett LE, Keck BM, Edwards EB, Novick RJ. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998; 351:24-7.
189. Maurer JR, Frost AE, Estenne M, Higenbottam T, Glanville AR. International guidelines for the selection of lung transplant candidates. The International Society for Heart and Lung Transplantation, the American Thoracic Society, the American Society of Transplant Physicians, the European Respiratory Society. Transplantation 1998; 66:951-6.
190. Ramsey SD, Patrick DL, Albert RK, Larson EB, Wood DE, Raghu G. The cost effectiveness of lung transplantation. A pilot study. University of Washington Medical Center Lung Transplant Study Group. Chest 1995; 108:1594-601.
191. Al MJ, Koopmanschap MA, van Enckevort PJ, Geertsma A, van der Bij W, de Boer WJ, et al. Cost effectiveness of lung transplantation in the Netherlands: a scenario analysis. Chest 1998; 113:124-30.
192. van Enckevort PJ, Koopmanschap MA, Tenvergert EM, Geertsma A, van der Bij W, de Boer WJ, et al. Lifetime costs of lung transplantation: estimation of incremental costs. Health Econ 1997; 6:479-89.
193. van Enckevort PJ, TenVergert EM, Bonsel GJ, Geertsma A, van der Bij W, de Boer WJ, et al. Technology assessment of the Dutch Lung Transplantation Program. Int J Technol Assess Healthcare1998; 14:344-56.
| Course |