| Course |
· Exacerbations of respiratory symptoms requiring medical intervention are important clinical events in COPD.
· The most common causes of an exacerbation are infection of the tracheobronchial tree and air pollution, but the cause of about one-third of severe exacerbations cannot be identified (Evidence B).
· Inhaled bronchodilators (particularly inhaled ß2-agonists and/or anticholinergics), theophylline, and systemic, preferably oral, glucocorticosteroids are effective treatments for acute exacerbations of COPD (Evidence A).
· Patients experiencing COPD exacerbations with clinical signs of airway infection (e.g., increased volume and change of color of sputum, and/or fever) may benefit from antibiotic treatment (Evidence B).
· Noninvasive intermittent positive pressure ventilation (NIPPV) in acute exacerbations improves blood gases and pH, reduces in-hospital mortality, decreases the need for invasive mechanical ventilation and intubation, and decreases the length of hospital stay (Evidence A).
COPD is often associated with acute exacerbations of symptoms1-4. In patients with Stage I: Mild COPD to Stage II: Moderate COPD, an exacerbation is associated with increased breathlessness, often accompanied by increased cough and sputum production, and may require medical attention outside of the hospital5. The need for medical intervention intensifies as the airflow limitation worsens. Exacerbations in Stage III: Severe COPD are associated with acute respiratory failure, representing a significant burden on the healthcare system. Hospital mortality of patients admitted for an acute exacerbation of COPD is approximately 10%, and the long-term outcome is poor. Mortality reaches 40% in one year6-9, and is even higher (up to 59%) for patients older than 65 years9. These figures vary from country to country depending on the healthcare system and the availability of intensive care unit (ICU) beds.
The most common causes of an exacerbation (Figure 5-4-1) are infection of the tracheobronchial tree10-14 and air pollution15, but the cause of about one-third of severe exacerbations cannot be identified6,16. The role of bacterial infections, once believed to be the main cause of COPD exacerbations, is controversial10-14,17-20. Conditions that may mimic an acute exacerbation include pneumonia, congestive heart failure, pneumothorax, pleural effusion, pulmonary embolism, and arrhythmia.
|
Figure 5-4-1. Common Causes of Acute Exacerbations of COPD |
|
|
Primary |
Secondary |
|
· Tracheobronchial infection · Air pollution. |
· Pneumonia · Pulmonary embolism · Pneumothorax · Rib fractures and chest trauma · Inappropriate use of sedatives, narcotics, beta-blocking agents · Right and/or left heart failure or arrhythmias |
Increased breathlessness, the main symptom of an exacerbation, is often accompanied by wheezing and chest tightness, increased cough and sputum, change of the color and/or tenacity of sputum, and fever. Exacerbations may also be accompanied by a number of nonspecific complaints, such as malaise, insomnia, sleepiness, fatigue, depression, and confusion. A decrease in exercise tolerance, fever, and/or new radiological anomalies suggestive of pulmonary disease may herald a COPD exacerbation. An increase in sputum volume and purulence points to a bacterial cause, as does prior history of chronic sputum production4,14.
Assessment of the severity of an acute exacerbation is based on the patient’s medical history before the exacerbation, symptoms, physical examination, lung function tests, arterial blood gas measurements, and other laboratory tests (Figure 5-4-2). Specific information is required on the frequency and severity of attacks of breathlessness and cough,
sputum volume and color, and limitation of daily activities. When available, prior tests of lung function and arterial blood gases are extremely useful for comparison with those made during the acute episode, as an acute change in these tests is more important than their absolute values. Thus, where possible, physicians should instruct their patients to bring the summary of their last evaluation when they come to the hospital with an exacerbation. In patients with very severe COPD, the most important sign of a severe exacerbation is a change in the alertness of the patient and this signals a need for immediate evaluation in the hospital.
|
Figure 5 4-2. Medical History an d Signs of Severity of Acute Exacerbations of COPD |
|
|
Medical History
|
Signs of Severity |
|
Duration of worsening or new symptoms |
Use of accessory respiratory muscles |
|
Number of previous episodes (exacerbations/hospitalizations |
Paradoxical chest wall movements |
|
Present treatment regimen |
Worsening or new on set central cyanosis |
|
|
Development of peripheral edema |
|
|
Hemodynamic instability |
|
|
Signs of right heart failure |
|
|
Reduced alertness |
Lung function tests. Even simple lung function tests can be difficult for a sick patient to perform properly. In general, a PEF < 100 L/min or an FEV1 < 1.00 L indicates a severe exacerbation21-23, except in patients with chronically severe airflow limitation. For instance, an FEV1 of 0.75 L, or a PaO2/FiO2 (FiO2 = fractional concentration of oxygen in dry inspired gas) of 33 kPa (24.5 mm Hg) may be well tolerated by a subject with severe COPD who copes with these values in stable conditions, whereas they may reflect a severe exacerbation for a subject with slightly higher values, e.g., an FEV1 of 0.90 L or a PaO2/FiO2 of 38 kPa (28.2 mm Hg) in stable conditions24.
Arterial blood gases. In the hospital, measurement of arterial blood gases is essential to assess the severity of an exacerbation. A PaO2 < 8.0 kPa (60 mm Hg) and/or SaO2 < 90% when breathing room air indicate respiratory failure. In addition, a PaO2 < 6.7 kPa (50 mm Hg), PaCO2 > 9.3 kPa (70 mm Hg), and pH < 7.30 point toward a life-threatening episode that needs ICU management25.
Chest X-ray and ECG. Chest radiographs (posterior/anterior plus lateral) are useful in identifying alternative diagnoses that can mimic the symptoms of an exacerbation. Although the history and physical signs can be confusing, especially when pulmonary hyperinflation masks coexisting cardiac signs, most problems are resolved by the chest X-ray and ECG. An ECG aids in the diagnosis of right heart hypertrophy, arrhythmias, and ischemic episodes. Pulmonary embolism can be very difficult to distinguish from an acute exacerbation, especially in severe COPD, because right ventricular hypertrophy and large pulmonary arteries lead to confusing ECG and radiographic results. Spiral CT scanning and angiography, and perhaps specific D-dimer assays, are the best tools presently available for the diagnosis of pulmonary embolism in patients with COPD, but ventilation-perfusion scanning is of no value. A low systolic blood pressure and an inability to increase the PaO2 above 8.0 kPa (60 mm Hg) despite high-flow oxygen also suggest pulmonary embolism. If there are strong indications that pulmonary embolism has occurred, it is best to treat for this along with the exacerbation.
Other laboratory tests. The whole blood count may identify polycythemia (hematocrit > 55%) or bleeding. White blood cell counts are usually not very informative. The presence of purulent sputum during an exacerbation of symptoms is sufficient indication for starting empirical antibiotic treatment. Streptococcus pneumoniae, Hemophiles influenzae, and Moraxella catarrhalis are the most common bacterial pathogens involved in COPD exacerbations. If an infectious exacerbation does not respond to the initial antibiotic treatment, a sputum culture and an antibiogram should be performed. Biochemical tests can reveal whether the cause of the exacerbation is an electrolyte disturbance(s) (hyponatremia, hypokalemia, etc.), a diabetic crisis, or poor nutrition (low proteins), and may suggest a metabolic acid-base disorder.
There is increasing interest in home care for end-stage COPD patients, although economic studies of home-care services have yielded mixed results. One study found that quality of life improved and hospital days per admission fell after a home-care program was instituted26, but a randomized controlled trial found that substituting home care for inpatient hospital care produced no better health outcomes while increasing costs27,28. A major outstanding issue is when to treat an exacerbation at home and when to hospitalize the patient.
The algorithm reported in Figure 5-4-3 may assist in the management of an acute exacerbation at home; a stepwise therapeutic approach is recommended29-32.
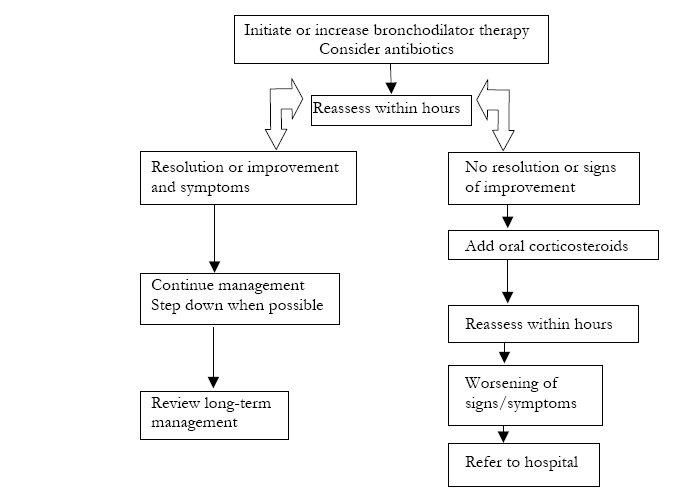
Home management of COPD exacerbations involves increasing the dose and/or frequency of existing bronchodilator therapy (Evidence A). If not already used, an anticholinergic can be added until the symptoms improve. In more severe cases, high-dose nebulizer therapy can be given on an as-needed basis for several days and if a suitable nebulizer is available. However, long-term use of nebulizer therapy after an acute episode is not routinely recommended.
Systemic glucocorticosteroids are beneficial in the management of acute exacerbations of COPD. They shorten recovery time and help to restore lung function more quickly33-35 (Evidence A). They should be considered in addition to bronchodilators if the patient’s baseline FEV1 is < 50% predicted. A dose of 40 mg prednisolone per day for 10 days is recommended (Evidence D).
Antibiotics are only effective when patients with worsening dyspnea and cough also have increased sputum volume and purulence4 (Evidence B). The choice of agents should reflect local patterns of antibiotic sensitivity among S. pneumoniae, H. influenzae, and M. catarrhalis.
The risk of dying from an acute exacerbation of COPD is closely related to the development of respiratory acidosis, the presence of significant co-morbidities, and the need for ventilatory support6. Patients lacking these features are not at high risk of dying, but those with severe underlying COPD often require hospitalization in any case. Attempts at managing such patients entirely in the community have met with only limited success28, but returning them to their homes with increased social support and a supervised medical care package after initial emergency room assessment has been much more successful36. Several randomized controlled trials have confirmed that this is a safe alternative to hospitalization, although it probably only applies to about 25% of COPD admissions. Savings on inpatient expenditures37 offset the additional costs of maintaining a community-based COPD nursing team. However, detailed cost-benefit analyses of these approaches are awaited.
Hospital assessment/admission should be considered for all patients who fit the criteria shown in Figure 5-4-4. Some patients need immediate admission to an intensive care unit (ICU). Admission of patients with severe COPD exacerbations to intermediate or special respiratory care units may be appropriate if personnel, skills, and equipment exist to identify and manage acute respiratory failure successfully.
|
Figure 5-4-4. Indications for Hospital Assessment or Admission for Acute Exacerbations of COPD |
|
· Marked increase in intensity of symptoms, such as sudden development of resting dyspnea |
|
· Severe background COPD |
|
· Onset of new physical signs (e.g., cyanosis, peripheral edema) |
|
· Failure of exacerbation to respond to initial medical management |
|
· Significant comorbidities. |
|
· Newly occurring arrhythmias |
|
· Diagnostic uncertainty |
|
· Older age. |
|
· Insufficient home support. |
|
Figure 5-4-5. Indications for ICU Admission of Patients with Acute Exacerbations of COPD |
|
· Severe dyspnea that responds inadequately to initial emergency therapy |
|
· Confusion, lethargy, coma |
|
· Persistent or worsening hypoxemia (Pa02 <6.7kPa, 50 mm Hg), and/or severe/worsening hypercapnia (PaC02 >9.3 kPa, 70 mm Hg), and/or severe/worsening respiratory acidosis (pH <7.30) |
The first actions when a patient reaches the emergency department are to provide controlled oxygen therapy and to determine whether the exacerbation is life threatening. If so, the patient should be admitted to the ICU immediately. Otherwise, the patient may be managed in the emergency department or hospital as detailed in Figure 5-4-6.
|
Figure 5-4-6 Management of Severe but Not Life-Threatening Exacerbations of COPD in the Emergency Department or the Hospital |
|
· Assess severity of symptoms, blood gases, chest X-ray. |
|
· Administer controlled oxygen therapy and repeat arterial blood gas measurement after 30 minutes. |
|
· Bronchodilators - Increase doses or frequency - Combine b2-agonists and anticholinergics - Use spacers or air-driven nebulizers. - Consider adding intravenous aminophylline, if needed. |
|
· Add oral or intravenous glucocorticosteroids |
|
· Consider antibiotics: - When signs of bacterial infection, oral or occasionally intravenous. - Consider noninvasive mechanical ventilation |
|
· At all times: - Monitor fluid balance and nutrition - Consider subcutaneous heparin. - Identify and treat associated conditions (e.g., heart failure, arrhythmias). - Closely monitor condition of the patient |
Controlled oxygen therapy. Oxygen therapy is the cornerstone of hospital treatment of COPD exacerbations. Adequate levels of oxygenation (PaO2 > 8.0 kPa, 60 mm Hg, or SaO2 > 90%) are easy to achieve in uncomplicated exacerbations, but CO2 retention can occur insidiously with little change in symptoms. Once oxygen is started, arterial blood gases should be checked 30 minutes later to ensure satisfactory oxygenation without CO2 retention or acidosis. Venturi masks are more accurate sources of controlled oxygen than are nasal prongs but are more likely to be removed by the patient.
Bronchodilator therapy. Short-acting inhaled ß2-agonists are usually the preferred bronchodilators for treatment of acute exacerbations of COPD30,31,38 (Evidence A). If a prompt response to these drugs does not occur, the addition of an anticholinergic is recommended, even though evidence concerning the effectiveness of this combination is rather controversial39,40. Despite its widespread clinical use, aminophylline’s role in the treatment of exacerbations of COPD remains controversial. Most studies of aminophylline have demonstrated minor improvements in lung volumes but also worsening of gas exchange and hypoxemia41,42. In more severe exacerbations, addition of an oral or intravenous methylxanthine to the treatment can be considered. However, close monitoring of serum theophylline is recommended to avoid the side effects of these drugs41,43-45.
Glucocorticosteroids. Oral or intravenous glucocorticosteroids are recommended as an addition to bronchodilator therapy (plus eventually antibiotics and oxygen therapy) in the hospital management of acute exacerbations of COPD33-35 (Evidence A). The exact dose that should be recommended is not known, but high doses are associated with a significant risk of side effects. Thirty to 40 mg of oral prednisolone daily for 10 to 14 days is a reasonable compromise between efficacy and safety (Evidence D). Prolonged treatment does not result in greater efficacy and increases the risk of side effects.
Antibiotics. Antibiotics are only effective when patients with worsening dyspnea and cough also have increased sputum volume and purulence4. The choice of agents should reflect local patterns of antibiotic sensitivity among S. pneumoniae, H. influenzae, and M. catarrhalis.
Ventilatory support. The primary objectives of mechanical support in patients with acute exacerbations in Stage III: Severe COPD are to decrease mortality and morbidity and to relieve symptoms. Ventilatory support includes both noninvasive mechanical ventilation using either negative or positive pressure devices, and invasive (conventional) mechanical ventilation by oro/naso-tracheal tube or tracheostomy.
Noninvasive mechanical ventilation. Noninvasive intermittent positive pressure ventilation (NIPPV) has been studied in many uncontrolled and five randomized controlled trials in acute respiratory failure46. The studies show consistently positive results with success rates of 80-85%47. Taken together they provide evidence that NIPPV increases pH, reduces PaCO2, reduces the severity of breathlessness in the first 4 hours of treatment, and decreases the length of hospital stay (Evidence A). More importantly, mortality - or its surrogate, intubation rate - is reduced by this intervention48-51. However, NIPPV is not appropriate for all patients, as summarized in Figure 5-4-747.
|
Figure 5 -4 -7. Selection and Exclusion Criteria for NIPPV |
|
Selection criteria (at least 2 should be present.) · Moderate to severe dyspnea with use of accessory muscles and paradoxical abdominal motion · Moderate to severe acidosis (pH 7.30-7.35) and hypercapnia (PaC02 >6.0-8.0 kPa, 45-60 mm Hg) · Respiratory frequency 25 breaths per minute |
|
Exclusion criteria (any may he present) · Respiratory arrest · Cardiovascular instability (hypotension, arrhythmias, myocardial infarction) · Somnolence, impaired mental status, uncooperative patient · High aspiration risk; viscous or copious secretions · Recent facial or gastroesophageal surgery · Craniofacial trauma, fixed nasopharyngeal abnormalities · Burns · Extreme obesity |
Invasive (conventional) mechanical ventilation. During exacerbations of COPD the events occurring within the lungs include bronchoconstriction, airway inflammation, increased mucous secretions, and loss of elastic recoil, all of which prevent the respiratory system from reaching its passive functional residual capacity at the end of expiration, enhancing dynamic hyperinflation52. As a result of these processes, an elastic threshold load, referred to as intrinsic or auto-positive end-expiratory pressure (PEEPi), is imposed on the inspiratory muscles at the beginning of inspiration and increases the work of breathing. For these reasons, patients who show impending acute respiratory failure and those with life-threatening acid-base status abnormalities and/or altered mental status despite aggressive pharmacologic therapy are likely to be the best candidates for invasive (conventional) mechanical ventilation. The indications for initiating mechanical ventilation during exacerbations of COPD are shown in Figure 5-4-8, the first being the commonest and most important reason. Figure 5-4-9 details the factors determining benefit from invasive ventilation. The three ventilatory modes most widely used are assisted-control ventilation, and pressure support ventilation alone or in combination with intermittent mandatory ventilation53.
Figure 5.4.8Indications for Invasive Mechanical Ventilation |
|
· Severe dyspnea with use of accessory muscles and paradoxical abdominal motion · Respiratory frequency > 35 breaths per minute · Life-threatening hypoxemia (PaO2 < 5.3 kPa, 40 mm Hg or PaO2/FiO2 < 200 mm Hg) · Severe acidosis (pH < 7.25) and hypercapnia (PaCO2 > 8.0 kPa, 60 mm Hg) · Respiratory arrest · Somnolence, impaired mental status · Cardiovascular complications (hypotension, shock, heart failure) · Other complications (metabolic abnormalities, sepsis, pneumonia, pulmonary embolism, barotrauma, massive pleural effusion) · NIPPV failure (or exclusion criteria) |
|
Figure 5.4-9 Factors Determining Benefit from Invasive Ventilation |
|
· Cultural attitudes toward chronic disability. · Expectations of therapy · Financial resources (especially the provision of ICU facilities). · Perceived likelihood of recovery · Customary medical practice. · Wishes, if known, of the patient.
|
The use of invasive ventilation in end-stage COPD patients is influenced by the likely reversibility of the precipitation event, the patient’s wishes, and the availability of intensive care facilities. Major hazards include the risk of ventilator-acquired pneumonia (especially when multi-resistant organisms are prevalent), barotrauma, and failure to wean to spontaneous ventilation. Contrary to some opinions, mortality among COPD patients with respiratory failure is no greater than mortality among patients ventilated for non-COPD causes.
A review of a large number of North American COPD patients ventilated for respiratory failure indicated an in-hospital mortality of 17-30%54. Further attrition over the next 12 months was particularly high among those patients who had poor lung function before ventilation (FEV1 < 30% predicted), had a non-respiratory comorbidity, or were housebound. Patients who did not have a previously diagnosed underlying disease, had respiratory failure due to a potentially reversible cause (such as an infection), or were relatively mobile and not using long-term oxygen did surprisingly well with ventilatory support. When possible, a clear statement of the patient’s own treatment wishes - an advance directive or “living will” - makes these difficult decisions much easier to resolve.
Weaning or discontinuation from mechanical ventilation can be particularly difficult and hazardous in patients with COPD. The most influential determinant of mechanical ventilatory dependency in these patients is the balance between the respiratory load and the capacity of the respiratory muscles to cope with this load55. By contrast, pulmonary gas exchange by itself is not a major difficulty in patients with COPD56-58. Weaning patients from the ventilator can be a very difficult and prolonged process and the best method remains a matter of debate59,68. Whether pressure support or a T-piece trial is used, weaning is shortened when a clinical protocol is adopted (Evidence A). Non-invasive ventilation has been applied to facilitate the weaning process in COPD patients with acute or chronic respiratory failure54. Compared with invasive pressure support ventilation, noninvasive intermittent positive pressure ventilation (NIPPV) during weaning shortened weaning time, reduced the stay in the intensive care unit, decreased the incidence of nosocomial pneumonia, and improved 60-day survival rates. Similar findings have been reported when NIPPV is used after extubation for hypercapnic respiratory failure61 (Evidence C).
Other measures. Further treatments that can be used in the hospital include: fluid administration (accurate monitoring of fluid balance is essential); nutrition (supplementary when the patient is too dyspneic to eat); low molecular heparin in immobilized, polycythemic, or dehydrated patients with or without a history of thromboembolic disease; and sputum clearance (by stimulating coughing and low-volume forced expirations as in home management). Manual or mechanical chest percussion and postural drainage may be beneficial in patients producing > 25 ml sputum per day or with lobar atelectasis.
Insufficient clinical data exist to establish the optimal duration of hospitalization in individual patients developing an exacerbation of COPD1,62,63. Consensus and limited data support the discharge criteria listed in Figure 5-4-10. Figure 5-4-11 provides items to include in a follow-up assessment 4 to 6 weeks after discharge from the hospital. Thereafter, follow-up is the same as for stable COPD, including supervising smoking cessation, monitoring the effectiveness of each drug treatment, and monitoring changes in spirometric parameters38.
|
Figure 5.4.10 Discharge Criteria for Patients with Acute Exacerbations of COPD |
|
· Inhaled B2-agonst therapy is required no more frequently than every 4 hrs · Patient, if previously By ambulatory, is able to walk across room. · Patient is able to eat and sleep without frequent awakening by dyspnea. · Patient has been clinically stable for 12-24 hrs. · Arterial blood gases have been stable for 12-24 hrs. · Patient (or home caregiver) fully understands correct use of medications. · Follow-up and home care arrangements have been completed (e.g., visiting nurse, oxygen delivery, meal provisions). · Patient, family, and physician are confident patient can manage successfully. |
|
Figure 5.4.11 Follow-up Assessment 4-6 Weeks After Discharge from Hospital for an Acute Exacerbation of COPD |
|
· Ability to cope in usual environment. · Measurement of FEV1. · Reassessment of inhaler technique. · Understanding of recommended treatment regimen. · Need for long-term oxygen therapy and/or home nebulizer (for patients with severe COPD. |
If hypoxemia developed during the exacerbation, arterial blood gases should be rechecked at discharge and at the follow-up visit. If the patient remains hypoxemic, long-term oxygen therapy should be instituted. Decisions about suitability for continuous domiciliary oxygen based on the severity of the acute hypoxemia during an exacerbation are frequently misleading.
The opportunities for prevention of future exacerbations should be reviewed before discharge, with particular attention to future influenza vaccination plans, knowledge about current therapy including inhaler technique641, 65, and how to recognize symptoms of exacerbations. Pharmacotherapy known to reduce the number of exacerbations should be considered. Social problems should be discussed and principal caregivers identified if the patient has a significant persisting disability.
1. Regueiro CR, Hamel MB, Davis RB, Desbiens N, Connors AF Jr, Phillips RS. A comparison of generalist and pulmonologist care for patients hospitalized with severe chronic obstructive pulmonary disease: resource intensity, hospital costs, and survival. SUPPORT investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Am J Med 1998; 105:366-72.
2. Gibson PG, Wlodarczyk JH, Wilson AJ, Sprogis A. Severe exacerbation of chronic obstructive airways disease: health resource use in general practice and hospital. J Qual Clin Pract 1998; 18:125-33.
3. Warren PM, Flenley DC, Millar JS, Avery A. Respiratory failure revisited: acute exacerbations of chronic bronchitis between 1961-68 and 1970-76. Lancet 1980; 1:467-70.
4. Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106:196-204.
5. Thompson AB, Mueller MB, Heires AJ, Bohling TL, Daughton D, Yancey SW, et al. Aerosolized beclomethasone in chronic bronchitis. Improved pulmonary function and diminished airway inflammation. Am Rev Respir Dis 1992; 146:389-95.
6. Connors AF Jr, Dawson NV, Thomas C, Harrell FE Jr, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) [published erratum appears in Am J Respir Crit Care Med 1997; 155:386]. Am J Respir Crit Care Med 1996; 154:959-67.
7. Kong GK, Belman MJ, Weingarten S. Reducing length of stay for patients hospitalized with exacerbation of COPD by using a practice guideline. Chest 1997; 111:89-94.
8. Fuso L, Incalzi RA, Pistelli R, Muzzolon R, Valente S, Pagliari G, et al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med 1995; 98:272-7.
9. Seneff MG, Wagner DP, Wagner RP, Zimmerman JE, Knaus WA. Hospital and 1-year survival of patients admitted to intensive care units with acute exacerbation of chronic obstructive pulmonary disease. JAMA 1995; 274:1852-7.
10. Macfarlane JT, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of aetiology and outcome of adult lower-respiratory-tract infections in the community. Lancet 1993; 341:511-4.
11. Smith CB, Kanner RE, Golden CA, Klauber MR, Renzetti AD Jr. Effect of viral infections on pulmonary function in patients with chronic obstructive pulmonary diseases. J Infect Dis 1980; 141:271-80.
12. Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care 1998; 157:1498-505.
13. Wilson R. The role of infection in COPD. Chest 1998; 113:242-8S.
14. Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 2000; 117:1638-45.
15. Anderson HR, Spix C, Medina S, Schouten JP, Castellsague J, Rossi G, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J 1997; 10:1064-71.
16. Chodosh S, McCarty J, Farkas S, Drehobl M, Tosiello R, Shan M, et al. Randomized, double-blind study of ciprofloxacin and cefuroxime axetil for treatment of acute bacterial exacerbations of chronic bronchitis. The Bronchitis Study Group. Clin Infect Dis 1998; 27:722-9.
17. Walsh EE, Falsey AR, Hennessey PA. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care 1999; 160:791-5.
18. Mogulkoc N, Karakurt S, Isalska B, Bayindir U, Celikel T, Korten V, et al. Acute purulent exacerbation of chronic obstructive pulmonary disease and Chlamydia pneumoniae infection. Am J Respir Crit Care Med 1999; 160:349-53.
19. Murphy TF, Sethi S, Klingman KL, Brueggemann AB, Doern GV. Simultaneous respiratory tract colonization by multiple strains of nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease: implications for antibiotic therapy. J Infect Dis 1999; 180:404-9.
20. Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest 1999; 116:40-6.
21. Emerman CL, Effron D, Lukens TW. Spirometric criteria for hospital admission of patients with acute exacerbation of COPD. Chest 1991; 99:595-9.
22. Emerman CL, Lukens TW, Effron D. Physician estimation of FEV1 in acute exacerbation of COPD. Chest 1994; 105:1709-12.
23. Emerman CL, Cydulka RK. Use of peak expiratory flow rate in emergency department evaluation of acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med 1996; 27:159-63.
24. Barbera JA, Roca J, Ferrer A, Felez MA, Diaz O, Roger N, et al. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1997; 10:1285-91.
25. Emerman CL, Connors AF, Lukens TW, Effron D, May ME. Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med 1989; 18:523-7.
26. Miles-Tapping C. Home care for chronic obstructive pulmonary disease: impact of the Iqaluit program. Arctic Med Res 1994; 53:163-75.
27. Shepperd S, Harwood D, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. II: cost minimisation analysis. BMJ 1998; 316:1791-6.
28. Shepperd S, Harwood D, Jenkinson C, Gray A, Vessey M, Morgan P. Randomised controlled trial comparing hospital at home care with inpatient hospital care. I: three month follow up of health outcomes. BMJ 1998; 316:1786-91.
29. Celli BR. Current thoughts regarding treatment of chronic obstructive pulmonary disease. Med Clin North Am 1996; 80:589-609.
30. Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 1995; 8:1398-420.
31. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152:S77-121.
32. Sachs FL. Chronic bronchitis. Clin Chest Med 1981; 2:79-89.
33. Thompson WH, Nielson CP, Carvalho P, Charan NB, Crowley JJ. Controlled trial of oral prednisone in outpatients with acute COPD exacerbation. Am J Respir Crit Care Med 1996; 154:407-12.
34. Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999; 354:456-60.
35. Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med, 1999; 340:1941-7.
36. Gravil JH, Al-Rawas OA, Cotton MM, Flanigan U, Irwin A, Stevenson RD. Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet 1998; 351:1853-5.
37. Soderstrom L, Tousignant P, Kaufman T. The health and cost effects of substituting home care for inpatient acute care: a review of the evidence. CMAJ 1999; 160:1151-5.
38. The COPD Guidelines Group of the Standards of Care Committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax 1997; 52 Suppl 5:S1-28.
39. Moayyedi P, Congleton J, Page RL, Pearson SB, Muers MF. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax 1995; 50:834-7.
40. Fernandez A, Munoz J, de la Calle B, Alia I, Ezpeleta A, de la Cal MA, et al. Comparison of one versus two bronchodilators in ventilated COPD patients. Intensive Care Med 1994; 20:199-202.
41. Barbera JA, Reyes A, Roca J, Montserrat JM, Wagner PD, Rodriguez-Roisin R. Effect of intravenously administered aminophylline on ventilation/perfusion inequality during recovery from exacerbations of chronic obstructive pulmonary disease. Am Rev Respir Dis 1992; 145:1328-33.
42. Mahon JL, Laupacis A, Hodder RV, McKim DA, Paterson NA, Wood TE, et al. Theophylline for irreversible chronic airflow limitation: a randomized study comparing n of 1 trials to standard practice. Chest 1999; 115:38-48.
43. Lloberes P, Ramis L, Montserrat JM, Serra J, Campistol J, Picado C, et al. Effect of three different bronchodilators during an exacerbation of chronic obstructive pulmonary disease. Eur Respir J 1988; 1:536-9.
44. Murciano D, Aubier M, Lecocguic Y, Pariente R. Effects of theophylline on diaphragmatic strength and fatigue in patients with chronic obstructive pulmonary disease. N Engl J Med 1984; 311:349-53.
45. Emerman CL, Connors AF, Lukens TW, May ME, Effron D. Theophylline concentrations in patients with acute exacerbation of COPD. Am J Emerg Med 1990; 8:289-92.
46. Meyer TJ, Hill NS. Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med 1994; 120:760-70.
47. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation--a consensus conference report. Chest 1999; 116:521-34.
48. Bott J, Carroll MP, Conway JH, Keilty SE, Ward EM, Brown AM, et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 1993; 341:1555-7.
49. Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 1995; 333:817-22.
50. Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS. Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 1995; 151:1799-806.
51. Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 2000; 355:1931-5.
52. Rossi A, Gottfried SB, Higgs BD, Zocchi L, Grassino A, Milic-Emili J. Respiratory mechanics in mechanically ventilated patients with respiratory failure. J Appl Physiol 1985; 58:1849-58.
53. Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000; 161:1450-8.
54. Keenan SP, Kernerman PD, Cook DJ, Martin CM, McCormack D, Sibbald WJ. Effect of noninvasive positive pressure ventilation on mortality in patients admitted with acute respiratory failure: a meta-analysis. Crit Care Med 1997; 25:1685-92.
55. Purro A, Appendini L, De Gaetano A, Gudjonsdottir M, Donner CF, Rossi A. Physiologic determinants of ventilator dependence in long-term mechanically ventilated patients. Am J Respir Crit Care Med 2000; 161:1115-23.
56. Torres A, Reyes A, Roca J, Wagner PD, Rodriguez-Roisin R. Ventilation-perfusion mismatching in chronic obstructive pulmonary disease during ventilator weaning. Am Rev Respir Dis 1989; 140:1246-50.
57. Beydon L, Cinotti L, Rekik N, Radermacher P, Adnot S, Meignan M, et al. Changes in the distribution of ventilation and perfusion associated with separation from mechanical ventilation in patients with obstructive pulmonary disease. Anesthesiology 1991; 75:730-8.
58. Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, et al. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 1998; 128:721-8.
59. Esteban A, Frutos F, Tobin MJ, Allia I, Solsona JF, Valverdu I, et al., for the Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med 1995;332:345-50.
60. Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 1994; 150:896-903
61. Hilbert G, Gruson D, Portel L, Gbikpi-Benissan G, Cardinaud JP. Noninvasive pressure support ventilation in COPD patients with postextubation hypercapnic respiratory insufficiency. Eur Respir J 1998; 11:1349-53.
62. Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159:158-64.
63. Mushlin AI, Black ER, Connolly CA, Buonaccorso KM, Eberly SW. The necessary length of hospital stay for chronic pulmonary disease. JAMA 1991; 266:80-3.
64. Stoller JK, Lange PA. Inpatient management of chronic obstructive pulmonary disease. Respir Care Clin N Am 1998; 4:425-38.
65. Peach H, Pathy MS. Follow-up study of disability among elderly patients discharged from hospital with exacerbations of chronic bronchitis. Thorax 1981; 36:585-9.
| Course |